J Korean Ophthalmol Soc.
2009 Mar;50(3):450-461. 10.3341/jkos.2009.50.3.450.
The Inhibitory Effect of TGF-beta Inhibitor on the Corneal Opacity After Corneal Laceration
- Affiliations
-
- 1Department of Ophthalmology, Inha University School of Medicine, Incheon, Korea. jhoh9707@hanmail.net
- KMID: 2111258
- DOI: http://doi.org/10.3341/jkos.2009.50.3.450
Abstract
-
PURPOSE: To evaluate the effects of TGF-beta inhibitor on the wound healing process after corneal laceration, and its inhibitory effect on corneal scar formation.
METHODS
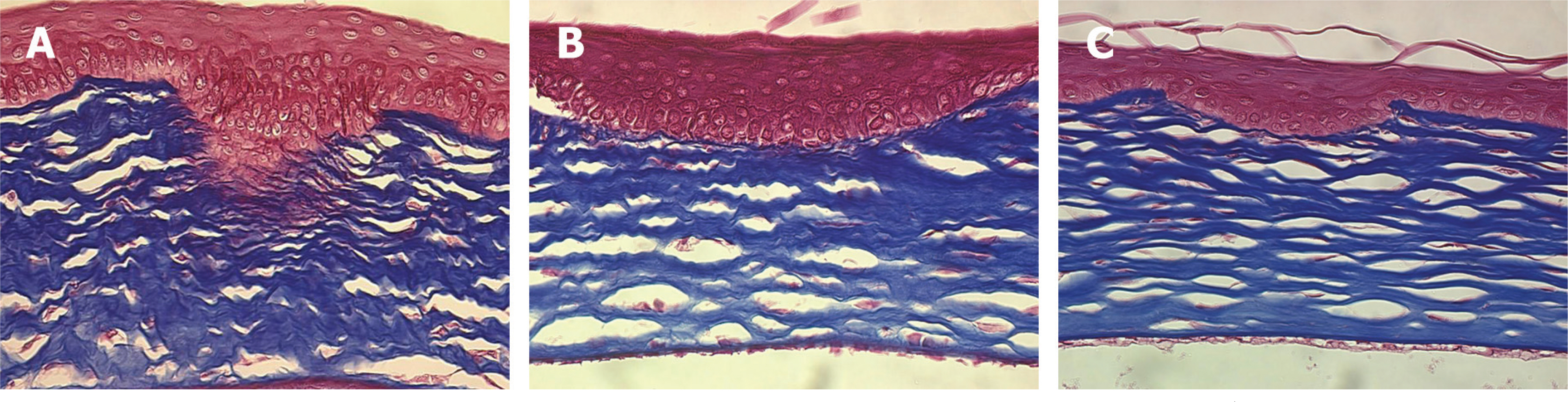
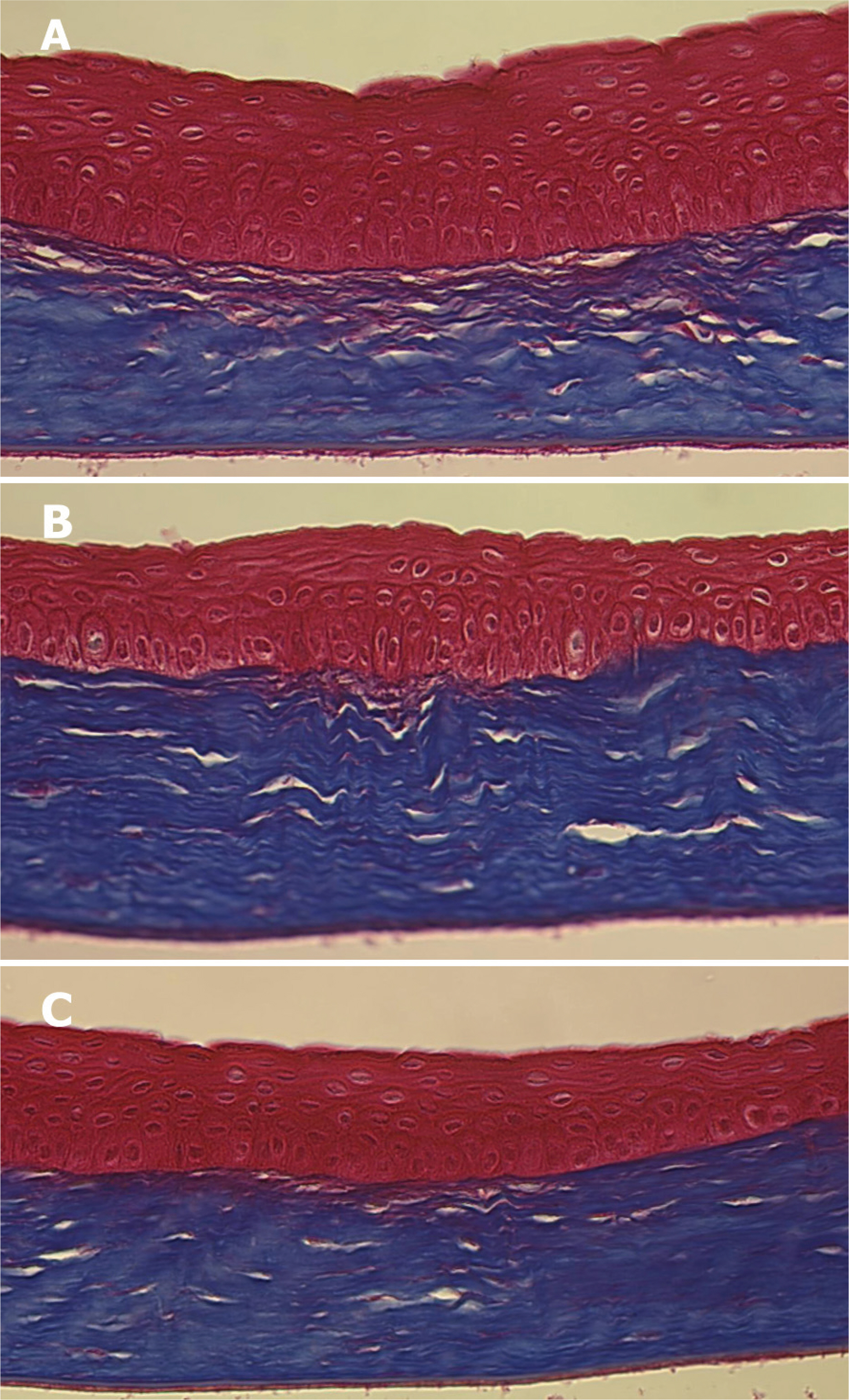
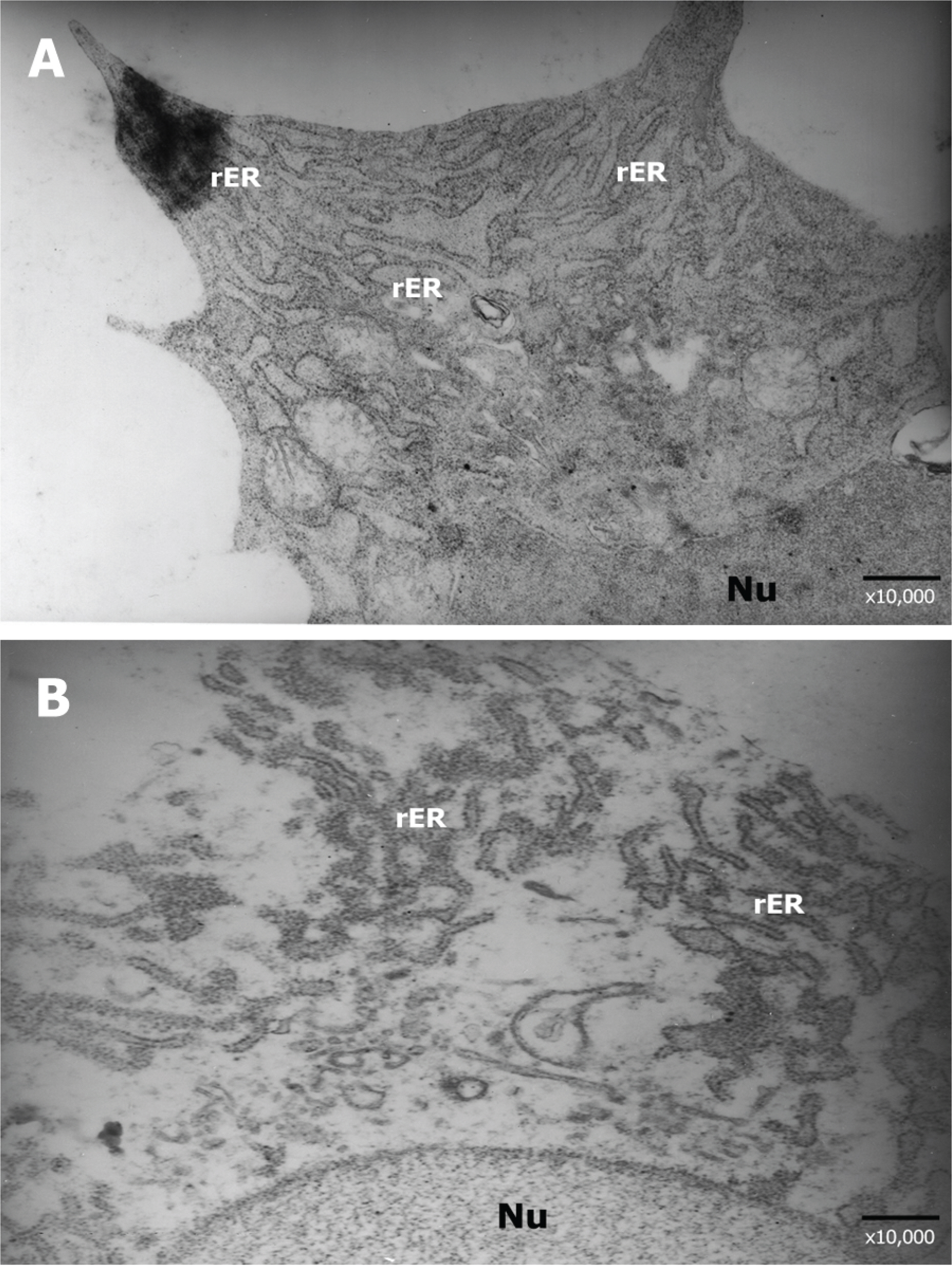
Forty Lewis rats were randomly divided into one control and three experimental groups (groups I, II, and III). After partial-thickness vertical linear corneal incision, a diluted solution with 10, 25, and 50 microgram of TGF-beta inhibitor was instilled into each eye of groups I, II, and III respectively. Corneal haze was measured by using slit-lamp biomicroscopic examination. Using histopathologic examination, we compared the number of stromal keratocytes and the arrangement of regenerated collagen fibers. We also performed immunohistochemistry to confirm the differential expression of fibronectin and alpha-smooth muscle actin in each group.
RESULTS
Group III showed less corneal haze and more regular arrangement of regenerated collagen fibers than the other groups. The number of stromal keratocytes and immunoreactivity to fibronectin and alpha-smooth muscle actin decreased as the dose of TGF-beta inhibitor increased.
CONCLUSIONS
TGF-beta inhibitor effectively reduced corneal haze during corneal healing processes after corneal laceration.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Topical Tranilast on Corneal Haze with the Pentacam® after Photorefractive Keratectomy
Sung In Kim, Tae Hoon Oh
J Korean Ophthalmol Soc. 2014;55(9):1277-1283. doi: 10.3341/jkos.2014.55.9.1277.
Reference
-
References
1. Krachmer JH, Mannis MJ, Holland EJ. Cornea. 2nd ed. Philadelphia: Elsevier & Mosby;2005. p. 115–46.2. Ohji M, SundarRaj N, Thoft RA. Transforming growth factor-beta stimulates collagen and fibronectin synthesis by human corneal stromal fibroblasts in vitro. Curr Eye Res. 1993; 12:703–9.3. Jester JV, Barry-Lane PA, Cavanagh HD, Petroll WM. Induction of alpha-smooth muscle actin expression and myofibroblast transfo-rmation in cultured corneal keratocytes. Cornea. 1996; 15:505–16.4. Fini ME, Girard MT, Matsubara M, Bartlett JD. Unique regulation of the matrix metalloproteinase, gelatinase B. Invest Ophthalmol Vis Sci. 1995; 36:622–33.5. Grant MB, Khaw PT, Schultz GS, et al. Effects of epidermal growth factor, fibroblast growth factor, and transforming growth factor-beta on corneal cell chemotaxis. Invest Ophthalmol Vis Sci. 1992; 33:3292–301.6. Desmouliere A, Rubbia-Brandt L, Grau G, Gabbiani G. Heparin induces alpha-smooth muscle actin expression in cultured fibroblasts and in granulation tissue myofibroblasts. Lab Invest. 1992; 67:716–26.7. Garana RM, Petroll WM, Chen WT, et al. Radial keratotomy. II. Role of the myofibroblast in corneal wound contraction. Invest Ophthalmol Vis Sci. 1992; 33:3271–82.8. Petroll WM, Cavanagh HD, Barry P, et al. Quantitative analysis of stress fiber orientation during corneal wound contraction. J Cell Sci. 1993; 104:353–63.
Article9. Shah M, Foreman DM, Ferguson MW. Neutralizing antibody to TFG-beta 1,2 reduces cutaneous scarring in adult rodents. J Cell Sci. 1994; 107:1137–57.10. Cordeiro MF, Mead A, Ali RR, et al. Novel antisense oligo-nucleotides targeting TGF-beta inhibit in vivo scarring and improve surgical outcome. Gene Ther. 2003; 10:59–71.11. Cordeiro MF, Gay JA, Khaw PT. Human anti-transforming growth factor-beta2 antibody: a new glaucoma anti-scarring agent. Invest Ophthalmol Vis Sci. 1999; 40:2225–34.12. Siriwardena D, Khaw PT, King AJ, et al. Human antitransforming growth factor beta (2) monoclonal antibody-a new modulator of wound healing in trabeculectomy: a randomized placebo controlled clinical study. Ophthalmology. 2002; 109:427–31.13. Moller-Pedersen T, Cavanagh HD, Petroll WM, Jester JV. Neutralizing antibody to TGF-beta modulates stromal fibrosis but not regression of photoablative effect following PRK. Curr Eye Res. 1998; 17:736–47.14. Mathers WD, Lemp MA. Theevolution of scarring following penetrating keratoplasty. Cavanogh HD, editor. The cornea: Trans-actions of the World congress on the Cornea III. New York: Raven Press;1988. Ⅱ:chap. 16.15. Goodman GL, Trokel SL, Stark WJ, et al. Corneal healing following laser refractive keratectomy. Arch Ophthalmol. 1989; 107:1799–803.
Article16. Thompson NL, Bazoberry F, Speir EH, et al. Transforming growth factor beta-1 in acute myocardial infarction in rats. Growth Factors. 1988; 1:91–9.
Article17. Wahl SM. Transforming growth factor beta (TGF-beta) in inflammation: a cause and a cure. J Clin Immunol. 1992; 12:61–74.18. Mita T, Yamashita H, Kaji Y, et al. Effects of transforming growth factor beta on corneal epithelial and stromal cell function in a rat wound healing model after excimer laser keratectomy. Graefes Arch Clin Exp Ophthalmol. 1998; 236:834–43.19. Hayashi K, Frangieh G, Wolf G, Kenyon KR. Expression of transforming growth factor-beta in wound healing of vitamin A-deficient rat corneas. Invest Ophthalmol Vis Sci. 1989; 30:239–47.20. Rochels R, Busse WD. In vivo evidence for the chemotactic activity of cyclooxygenase- and lipoxygenase-dependent compounds using a corneal implantation technique. Ophthalmic Res. 1984; 16:194–7.
Article21. Azar DT, Hahn TW, Jain S, et al. Matrix metalloproteinases are expressed during wound healing after excimer laser keratectomy. Cornea. 1996; 15:18–24.
Article22. Rieck P, Assouline M, Savoldelli M, et al. Recombinant human basic fibroblast growth factor (Rh-bFGF) in three different wound models in rabbits: corneal wound healing effect and pharmacology. Exp Eye Res. 1992; 54:987–98.
Article23. Ohno K, Mitooka K, Nelson LR, et al. Keratocyte activation and apoptosis in transplanted human corneas in a xenograft model. Invest Ophthalmol Vis Sci. 2002; 43:1025–31.24. Fini ME. Keratocyte and fibroblast phenotypes in the repairing cornea. Prog Retin Eye Res. 1999; 18:529–51.
Article25. Eckes B, Kessler D, Aumailley M, Krieg T. Interactions of fibroblasts with the extracellular matrix: implications for the understanding of fibrosis. Springer Semin Immunopathol. 1999; 21:415–29.
Article26. Davison PF, Galbavy EJ. Fluorescent dyes demonstrate the uniform expansion of the growing rabbit cornea. Invest Ophthalmol Vis Sci. 1985; 26:1202–9.27. Davison PF, Galbavy EJ. Connective tissue remodeling in corneal and scleral wounds. Invest Ophthalmol Vis Sci. 1986; 27:1478–84.28. Tuft SJ, Zabel RW, Marshall J. Corneal repair following keratectomy. A comparison between conventional surgery and laser photoablation. Invest Ophthalmol Vis Sci. 1989; 30:1769–77.29. Jain S, Hahn TW, McCally RL, Azar DT. Antioxidants reduce corneal light scattering after excimer keratectomy in rabbits. Lasers Surg Med. 1995; 17:160–5.
Article30. Kim TI, Lee SY, Pak JH, et al. Mitomycin C, ceramide, and 5-fluorouracil inhibit corneal haze and apoptosis after PRK. Cornea. 2006; 25:55–60.
Article31. Carones F, Vigo L, Scandola E, Vacchini L. Evaluation of the prophylactic use of mitomycin-C to inhibit haze formation after photorefractive keratectomy. J Cataract Refract Surg. 2002; 28:2088–95.
Article32. Safianik B, Ben-Zion I, Garzozi HJ. Serious corneoscleral complications after pterygium excision with mitomycin C. Br J Ophthalmol. 2002; 86:357–8.
Article33. Hayasaka S, Iwasa Y, Nagaki Y, et al. Late complications after pterygium excision with high dose mitomycin C instillation. Br J Ophthalmol. 2000; 84:1081–2.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Evaluation of Traumatic Cataract with Corneal Laceration
- A Case of Spontaneous Regression of Schnyder's Crystalline Corneal Dystrophy
- Comparison of Corneal Haze according to the Success of Corneal Epithelial Flap after Laser Subepithelial Keratomileusis
- Hypohidrotic Ectodermal Dysplasia with Congenital Cataract and Corneal Opacity: Report of a Case
- Cataract Operation in Eyes with Corneal Opacity