J Periodontal Implant Sci.
2011 Dec;41(6):263-272. 10.5051/jpis.2011.41.6.263.
A comprehensive review of techniques for biofunctionalization of titanium
- Affiliations
-
- 1Institute of Biomaterials and Bioengineering, Tokyo Medical and Dental University, Tokyo, Japan. hanawa.met@tmd.ac.jp
- KMID: 2094722
- DOI: http://doi.org/10.5051/jpis.2011.41.6.263
Abstract
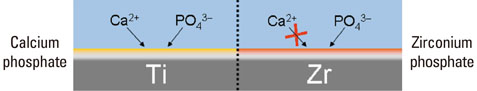
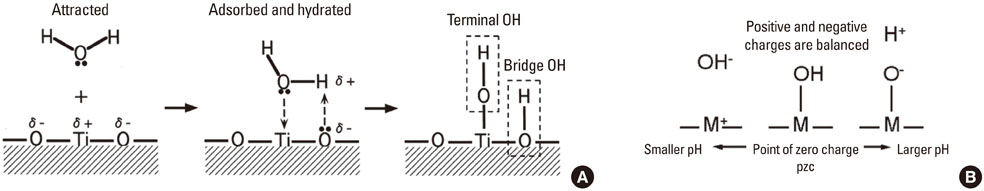
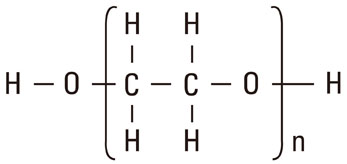
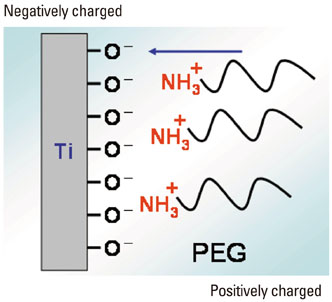
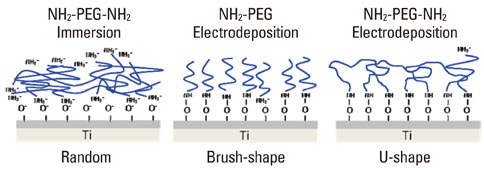
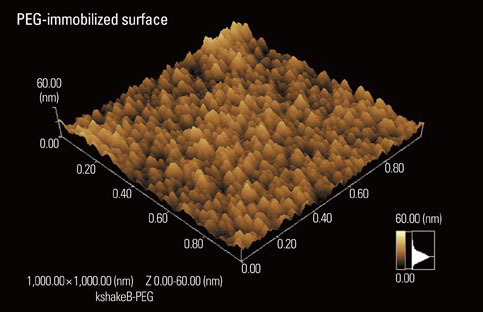
- A number of surface modification techniques using immobilization of biofunctional molecules of Titanium (Ti) for dental implants as well as surface properties of Ti and Ti alloys have been developed. The method using passive surface oxide film on titanium takes advantage of the fact that the surface film on Ti consists mainly of amorphous or low-crystalline and non-stoichiometric TiO2. In another method, the reconstruction of passive films, calcium phosphate naturally forms on Ti and its alloys, which is characteristic of Ti. A third method uses the surface active hydroxyl group. The oxide surface immediately reacts with water molecules and hydroxyl groups are formed. The hydroxyl groups dissociate in aqueous solutions and show acidic and basic properties. Several additional methods are also possible, including surface modification techniques, immobilization of poly(ethylene glycol), and immobilization of biomolecules such as bone morphogenetic protein, peptide, collagen, hydrogel, and gelatin.
Keyword
MeSH Terms
-
Alloys
Bone Morphogenetic Proteins
Calcium
Calcium Phosphates
Collagen
Dental Implants
Electroplating
Gelatin
Hydrogel
Imidazoles
Immobilization
Nitro Compounds
Surface Properties
Titanium
Alloys
Bone Morphogenetic Proteins
Calcium
Calcium Phosphates
Collagen
Dental Implants
Gelatin
Hydrogel
Imidazoles
Nitro Compounds
Titanium
Figure
Reference
-
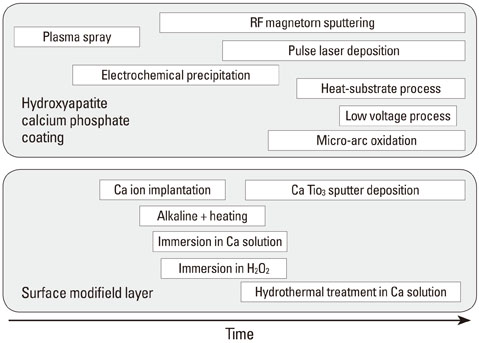
1. Yang Y, Kim KH, Ong JL. A review on calcium phosphate coatings produced using a sputtering process--an alternative to plasma spraying. Biomaterials. 2005. 26:327–337.
Article2. Kim KH, Ramaswamy N. Electrochemical surface modification of titanium in dentistry. Dent Mater J. 2009. 28:20–36.
Article3. Kelly EJ. Electrochemical-behavior of titanium. Mod Asp Electrochem. 1982. 14:319–424.4. Sundgren JE, Bodo P, Lundstrom I. Auger-electron spectroscopic studies of the interface between human-tissue and implants of titanium and stainless-steel. J Colloid Interface Sci. 1986. 110:9–20.
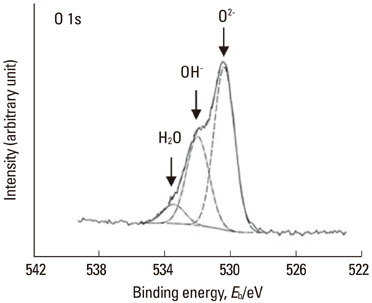
Article5. Hanawa T, Ota M. Calcium phosphate naturally formed on titanium in electrolyte solution. Biomaterials. 1991. 12:767–774.
Article6. Hanawa T. Davies JE, editor. Titanium and Its Oxide Film: a substrate for formation of apatite. The bone-biomaterial interface. 1991. Toronto: University of Toronto Press;49–61.7. Hanawa T, Ota M. Characterization of surface-film formed on titanium in electrolyte using XPS. Appl Surf Sci. 1992. 55:269–276.
Article8. Hanawa T, Okuno O, Hamanaka H. Compositional change in surface of TI-ZR alloys in artificial bioliquid. J Jpn Inst Met. 1992. 56:1168–1173.
Article9. Hiromoto S, Hanawa T, Asami K. Composition of surface oxide film of titanium with culturing murine fibroblasts L929. Biomaterials. 2004. 25:979–986.
Article10. Tsutsumi Y, Nishimura D, Doi H, Nomura N, Hanawa T. Difference in surface reactions between titanium and zirconium in Hanks' solution to elucidate mechanism of calcium phosphate formation on titanium using XPS and cathodic polarization. Mater Sci Eng C-Biom Supramol Syst. 2009. 29:1702–1708.
Article11. Parfitt GD. The surface of titanium dioxide. Prog Surf Membr Sci. 1976. 11:181–226.
Article12. Westall J, Hohl H. A comparison of electrostatic models for the oxide/solution interface. Adv Colloid Interface Sci. 1980. 12:265–294.
Article13. Healy TW, Fuerstenau DW. The oxide-water interface-Interreaction of the zero point of charge and the heat of immersion. J Colloid Sci. 1965. 20:376–386.
Article14. Boehm HP. Acidic and basic properties of hydroxylated metal oxide surfaces. Discuss Faraday Soc. 1971. (52):264–275.
Article15. Mahato RI. Biomaterials for delivery and targeting of proteins and nucleic acids. 2005. Boca Raton: CRC Press.16. Kenausis GL, Voros J, Elbert DL, Huang NP, Hofer R, Ruiz-Taylor L, et al. Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: attachment mechanism and effects of polymer architecture on resistance to protein adsorption. J Phys Chem. 2000. 104:3298–3309.
Article17. Huang NP, Michel R, Voros J, Textor M, Hofer R, Rossi A, et al. Poly(L-lysine)-g-poly(ethylene glycol) layers on metal oxide surfaces: surface-analytical characterization and resistance to serum and fibrinogen adsorption. Langmuir. 2001. 17:489–498.
Article18. Huang NP, Csucs G, Emoto K, Nagasaki Y, Kataoka K, Textor M, et al. Covalent attachment of novel poly(ethylene glycol)-poly(DL-lactic acid) copolymeric micelles to TiO2 surfaces. Langmuir. 2002. 18:252–258.
Article19. Zhang F, Kang ET, Neoh KG, Wang P, Tan KL. Surface modification of stainless steel by grafting of poly(ethylene glycol) for reduction in protein adsorption. Biomaterials. 2001. 22:1541–1548.
Article20. Ito Y, Hasuda H, Sakuragi M, Tsuzuki S. Surface modification of plastic, glass and titanium by photoimmobilization of polyethylene glycol for antibiofouling. Acta Biomater. 2007. 3:1024–1032.
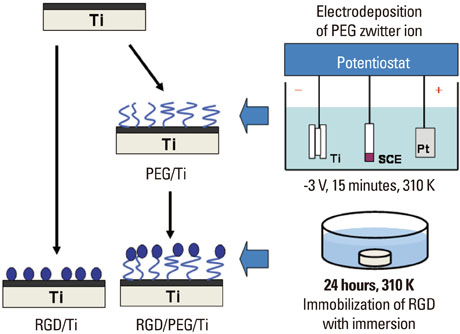
Article21. Tanaka Y, Doi H, Iwasaki Y, Hiromoto S, Yoneyama T, Asami K, et al. Electrodeposition of amine-terminated poly (ethylene glycol) to titanium surface. Mater Sci Eng C-Biom Supramol Syst. 2007. 27:206–212.
Article22. Tanaka Y, Doi H, Kobayashi E, Yoneyama T, Hanawa T. Determination of the immobilization manner of amine-terminated poly(ethylene glycol) electrodeposited on a titanium surface with XPS and GD-OES. Mater Trans. 2007. 48:287–292.
Article23. Tanaka Y, Saito H, Tsutsumi Y, Doi H, Imai H, Hanawa T. Active hydroxyl groups on surface oxide film of titanium, 316L stainless steel, and cobalt-chromium-molybdenum alloy and its effect on the immobilization of poly(ethylene glycol). Mater Trans. 2008. 49:805–811.
Article24. Tanaka Y, Matsuo Y, Komiya T, Tsutsumi Y, Doi H, Yoneyama T, et al. Characterization of the spatial immobilization manner of poly(ethylene glycol) to a titanium surface with immersion and electrodeposition and its effects on platelet adhesion. J Biomed Mater Res A. 2010. 92:350–358.
Article25. Tanaka Y, Matin K, Gyo M, Okada A, Tsutsumi Y, Doi H, et al. Effects of electrodeposited poly(ethylene glycol) on biofilm adherence to titanium. J Biomed Mater Res A. 2010. 95:1105–1113.
Article26. Balachnder N, Sukenik CN. Monolayer transformation by nucleophilic-substitution- applications to the creation of new monolayer assemblies. Langmuir. 1990. 6:1621–1627.
Article27. Bain CD, Troughton EB, Tao YT, Evall J, Whitesides GM, Nuzzo RG. Formation of monolayer films by the spontaneous assembly of organic thiols from solution onto gold. J Am Chem Soc. 1989. 111:321–335.
Article28. Dubois LH, Nuzzo RG. Synthesis, structure, and properties of model organic-surfaces. Ann Rev Phys Chem. 1992. 43:437–463.29. Ulman A. Formation and structure of self-assembled monolayers. Chem Rev. 1996. 96:1533–1554.
Article30. Xiao SJ, Textor M, Spencer ND, Sigrist H. Covalent attachment of cell-adhesive, (Arg-Gly-Asp)-containing peptides to titanium surfaces. Langmuir. 1998. 14:5507–5516.
Article31. Gawalt ES, Avaltroni MJ, Danahy MP, Silverman BM, Hanson EL, Midwood KS, et al. Bonding organics to Ti alloys: facilitating human osteoblast attachment and spreading on surgical implant materials. Langmuir. 2003. 19:200–204.
Article32. Brovelli D, Hahner G, Ruis L, Hofer R, Kraus G, Waldner A, et al. Highly oriented, self-assembled alkanephosphate monolayers on tantalum(V) oxide surfaces. Langmuir. 1999. 15:4324–4327.
Article33. Textor M, Ruiz L, Hofer R, Rossi K, Feldman K, Hahner G, et al. Structural chemistry of self-assembled monolayers of octadecylphosphoric acid on tantalum oxide surfaces. Langmuir. 2000. 16:3257–3271.
Article34. Fang JL, Wu NJ, Wang ZW, Li Y. XPS, AES and Raman studies of an antitarnish film on tin. Corrosion. 1991. 47:169–173.
Article35. Van Alsten JG. Self-assembled monolayers on engineering metals: structure, derivatization, and utility. Langmuir. 1999. 15:7605–7614.
Article36. Gawalt ES, Avaltroni MJ, Koch N, Schwartz J. Self-assembly and bonding of alkanephosphonic acids on the native oxide surface of titanium. Langmuir. 2001. 17:5736–5738.
Article37. Verrier S, Pallu S, Bareille R, Jonczyk A, Meyer J, Dard M, et al. Function of linear and cyclic RGD-containing peptides in osteoprogenitor cells adhesion process. Biomaterials. 2002. 23:585–596.
Article38. Reyes CD, Petrie TA, Burns KL, Schwartz Z, García AJ. Biomolecular surface coating to enhance orthopaedic tissue healing and integration. Biomaterials. 2007. 28:3228–3235.
Article39. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002. 110:673–687.40. Bagno A, Piovan A, Dettin M, Chiarion A, Brun P, Gambaretto R, et al. Human osteoblast-like cell adhesion on titanium substrates covalently functionalized with synthetic peptides. Bone. 2007. 40:693–699.
Article41. Elmengaard B, Bechtold JE, Søballe K. In vivo study of the effect of RGD treatment on bone ongrowth on press-fit titanium alloy implants. Biomaterials. 2005. 26:3521–3526.
Article42. Rammelt S, Illert T, Bierbaum S, Scharnweber D, Zwipp H, Schneiders W. Coating of titanium implants with collagen, RGD peptide and chondroitin sulfate. Biomaterials. 2006. 27:5561–5571.
Article43. Auernheimer J, Zukowski D, Dahmen C, Kantlehner M, Enderle A, Goodman SL, et al. Titanium implant materials with improved biocompatibility through coating with phosphonate-anchored cyclic RGD peptides. Chembiochem. 2005. 6:2034–2040.
Article44. Ferris DM, Moodie GD, Dimond PM, Gioranni CW, Ehrlich MG, Valentini RF. RGD-coated titanium implants stimulate increased bone formation in vivo. Biomaterials. 1999. 20:2323–2331.
Article45. Xiao SJ, Textor M, Spencer ND, Wieland M, Keller B, Sigrist H. Immobilization of the cell-adhesive peptide Arg-Gly-Asp-Cys (RGDC) on titanium surfaces by covalent chemical attachment. J Mater Sci Mater Med. 1997. 8:867–872.46. Silverman BM, Wieghaus KA, Schwartz J. Comparative properties of siloxane vs phosphonate monolayers on a key titanium alloy. Langmuir. 2005. 21:225–228.
Article47. Schwartz J, Avaltroni MJ, Danahy MP, Silverman BM, Hanson EL, Schwarzbauer JE, et al. Cell attachment and spreading on metal implant materials. Mater Sci Eng C-Biom Supramol Syst. 2003. 23:395–400.
Article48. Tanaka Y, Saito H, Tsutsumi Y, Doi H, Nomura N, Imai H, et al. Effect of pH on the interaction between zwitterions and titanium oxide. J Colloid Interface Sci. 2009. 330:138–143.
Article49. Oya K, Tanaka Y, Saito H, Kurashima K, Nogi K, Tsutsumi H, et al. Calcification by MC3T3-E1 cells on RGD peptide immobilized on titanium through electrodeposited PEG. Biomaterials. 2009. 30:1281–1286.
Article50. Park JW, Kurashima K, Tustusmi Y, An CH, Suh JY, Doi H, et al. Bone healing of commercial oral implants with RGD immobilization through electrodeposited poly(ethylene glycol) in rabbit cancellous bone. Acta Biomater. 2011. 7:3222–3229.
Article51. Yamamichi N, Pugdee K, Chang WJ, Lee SY, Yoshinari M, Hayakawa T, et al. Gene expression monitoring in osteoblasts on titanium coated with fibronectin-derived peptide. Dent Mater J. 2008. 27:744–750.
Article52. Urist MR. Bone: formation by autoinduction. Science. 1965. 150:893–899.
Article53. Lee YM, Nam SH, Seol YJ, Kim TI, Lee SJ, Ku Y, et al. Enhanced bone augmentation by controlled release of recombinant human bone morphogenetic protein-2 from bioabsorbable membranes. J Periodontol. 2003. 74:865–872.
Article54. Wikesjö UM, Lim WH, Thomson RC, Cook AD, Wozney JM, Hardwick WR. Periodontal repair in dogs: evaluation of a bioabsorbable space-providing macroporous membrane with recombinant human bone morphogenetic protein-2. J Periodontol. 2003. 74:635–647.
Article55. Seol YJ, Park YJ, Lee SC, Kim KH, Lee JY, Kim TI, et al. Enhanced osteogenic promotion around dental implants with synthetic binding motif mimicking bone morphogenetic protein (BMP)-2. J Biomed Mater Res A. 2006. 77:599–607.
Article56. Puleo DA, Kissling RA, Sheu MS. A technique to immobilize bioactive proteins, including bone morphogenetic protein-4 (BMP-4), on titanium alloy. Biomaterials. 2002. 23:2079–2087.
Article57. Nanci A, Wuest JD, Peru L, Brunet P, Sharma V, Zalzal S, et al. Chemical modification of titanium surfaces for covalent attachment of biological molecules. J Biomed Mater Res. 1998. 40:324–335.
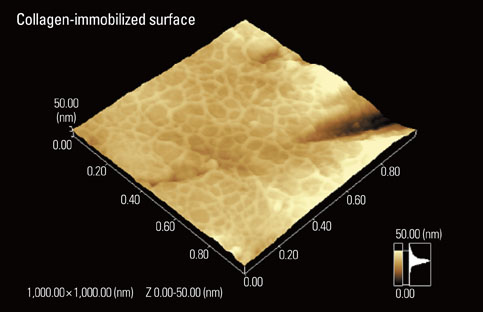
Article58. Nagai M, Hayakawa T, Fukatsu A, Yamamoto M, Fukumoto M, Nagahama F, et al. In vitro study of collagen coating of titanium implants for initial cell attachment. Dent Mater J. 2002. 21:250–260.
Article59. Viornery C, Guenther HL, Aronsson BO, Péchy P, Descouts P, Grätzel M. Osteoblast culture on polished titanium disks modified with phosphonic acids. J Biomed Mater Res. 2002. 62:149–155.
Article60. Chang WJ, Ou KL, Lee SY, Chen JY, Abiko Y, Lin CT, et al. Type I collagen grafting on titanium surfaces using low-temperature glow discharge. Dent Mater J. 2008. 27:340–346.
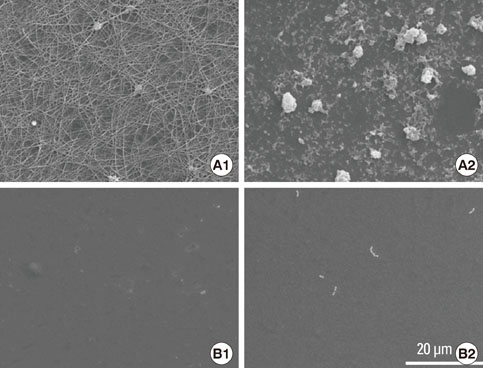
Article61. Kamata H, Suzuki S, Tanaka Y, Tsutsumi Y, Doi H, Nomura N, et al. Effects of pH, potential, and deposition time on the durability of collagen electrodeposited to titanium. Mater Trans. 2011. 52:81–89.
Article62. Pugdee K, Shibata Y, Yamamichi N, Tsutsumi H, Yoshinari M, Abiko Y, et al. Gene expression of MC3T3-E1 cells on fibronectin-immobilized titanium using tresyl chloride activation technique. Dent Mater J. 2007. 26:647–655.
Article63. Abe Y, Hiasa K, Takeuchi M, Yoshida Y, Suzuki K, Akagawa Y. New surface modification of titanium implant with phospho-amino acid. Dent Mater J. 2005. 24:536–540.
Article64. Cadotte AJ, DeMarse TB. Poly-HEMA as a drug delivery device for in vitro neural networks on micro-electrode arrays. J Neural Eng. 2005. 2:114–122.
Article65. Belkas JS, Munro CA, Shoichet MS, Johnston M, Midha R. Long-term in vivo biomechanical properties and biocompatibility of poly(2-hydroxyethyl methacrylate-co-methyl methacrylate) nerve conduits. Biomaterials. 2005. 26:1741–1749.
Article66. Indolfi L, Causa F, Netti PA. Coating process and early stage adhesion evaluation of poly(2-hydroxy-ethyl-methacrylate) hydrogel coating of 316L steel surface for stent applications. J Mater Sci Mater Med. 2009. 20:1541–1551.
Article67. Fenelon AM, Breslin CB. The electropolymerization of pryrole at a CuNi electrode: corrosion protection properties. Corros Sci. 2003. 45:2837–2850.
Article68. Mengoli G. Feasibility of polymer film coatings through electroinitiated polymerization in aqueous medium. Adv Polym Sci. 1979. 33:1–31.
Article69. De Giglio E, Guascito MR, Sabbatin L, Zambonin G. Electropolymerization of pyrrole on titanium substrates for the future development of new biocompatible surfaces. Biomaterials. 2001. 22:2609–2616.
Article70. Rammelt U, Nguyen PT, Plieth W. Corrosion protection by ultrathin films of conducting polymers. Electrochimica Acta. 2003. 48:1257–1262.
Article71. De Giglio E, De Gennaro L, Sabbatini L, Zambonin G. Analytical characterization of collagen- and/or hydroxyapatite-modified polypyrrole films electrosynthesized on Ti-substrates for the development of new bioactive surfaces. J Biomater Sci Polym Ed. 2001. 12:63–76.
Article72. De Giglio E, Cometa S, Satriano C, Sabbatini L, Zambonin PG. Electrosynthesis of hydrogel films on metal substrates for the development of coatings with tunable drug delivery performances. J Biomed Mater Res A. 2009. 88:1048–1057.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Comparison of Titanium Micro Mesh(R) with Titanium Mesh Screen 1.3(R) in the Reconstruction of Medial Orbital wall Fracture
- A histologic comparative study of loaded and unloaded titanium implants
- A comparative histologic study of bone-implant interface to the titanium root formed implants in the Mx, Mn
- A study on shear bond strength of interface between bone and titanium plasma sprayed IMZ implant in rabbits
- Techniques for dental implant nanosurface modifications