Korean J Hematol.
2010 Jun;45(2):120-126. 10.5045/kjh.2010.45.2.120.
Reduced-dose craniospinal radiotherapy followed by high-dose chemotherapy and autologous stem cell rescue for children with newly diagnosed high-risk medulloblastoma or supratentorial primitive neuroectodermal tumor
- Affiliations
-
- 1Department of Pediatrics, College of Medicine, Hanyang University, Seoul, Korea.
- 2Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 3Department of Pediatrics, College of Medicine, Yeungnam University, Daegu, Korea. johah@med.yu.ac.kr
- 4Department of Pediatrics, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Pediatrics, University of Ulsan, Asan Medical Center, Seoul, Korea.
- 6Department of Pediatrics, College of Medicine, Chungnam National University, Daejeon, Korea.
- 7Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 8Department of Radiation Oncology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- 9Department of Neurosurgery, Seoul National University College of Medicine, Korea.
- 10Department of Neurosurgery, University of Ulsan, Asan Medical Center, Seoul, Korea.
- 11Institute for Clinical Research, College of Medicine, CHA Medical University, Seongnam, Korea.
- KMID: 2083545
- DOI: http://doi.org/10.5045/kjh.2010.45.2.120
Abstract
- BACKGROUND
In this study, we investigated the effects of reduced-dose craniospinal radiotherapy (CSRT) followed by tandem high-dose chemotherapy (HDCT) with autologous stem cell rescue (ASCR) in children with a newly diagnosed high-risk medulloblastoma (MB) or supratentorial primitive neuroectodermal tumor (sPNET).
METHODS
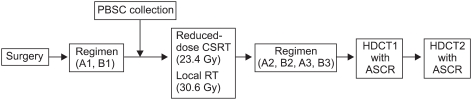
Between March 2005 and April 2007, patients older than 3 years with a newly diagnosed high-risk MB or sPNET were enrolled. The patients received two cycles of pre-RT chemotherapy consisting of cisplatin, etoposide, vincristine, and cyclophosphamide (cycle A), and carboplatin, etoposide, vincristine, and ifosphamide (cycle B), followed by CSRT with 23.4 Gy and local RT with 30.6 Gy. After four cycles of post-RT chemotherapy (cycles A, B, A, and B), tandem double HDCT with ASCR was performed.
RESULTS
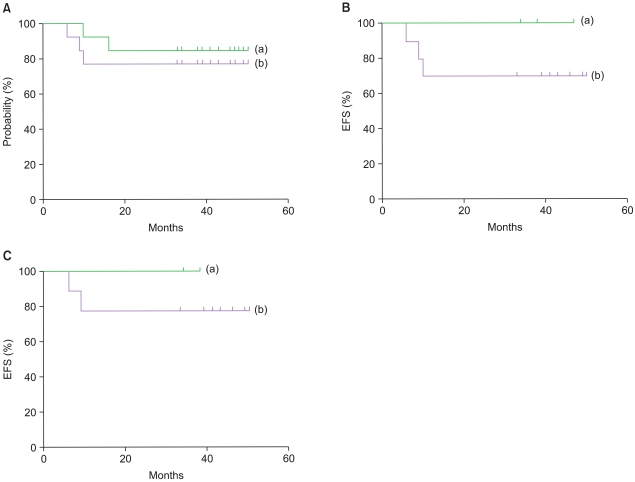
A total of 13 patients (MB=11, sPNET=2) were enrolled. Of these, one patient progressed, one patient died of septic shock after the second cycle of B, and one patient relapsed after the third cycle of B. The 3-year event-free survival (EFS) rate of the patients intended for HDCT was 76.9%, whereas the 3-year EFS rate of the patients who received HDCT was 100%. No treatment-related mortality occurred during HDCT.
CONCLUSION
Although the follow-up period was short and the patient cohort was small in size, the results of this study are encouraging. The limited toxicity and favorable EFS rate observed in children treated with reduced-dose CSRT followed by HDCT and ASCR warrant further exploration in a larger study population.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
The role of chemotherapy in the treatment of pediatric brain tumors
Jae-Wook Lee, Nack-Gyun Chung
J Korean Med Assoc. 2012;55(5):420-429. doi: 10.5124/jkma.2012.55.5.420.
Reference
-
1. Zeltzer PM, Boyett JM, Finlay JL, et al. Metastasis stage, adjuvant treatment, and residual tumor are prognostic factors for medulloblastoma in children: conclusions from the Children's Cancer Group 921 randomized phase III study. J Clin Oncol. 1999; 17:832–845. PMID: 10071274.
Article2. Fouladi M, Gilger E, Kocak M, et al. Intellectual and functional outcome of children 3 years old or younger who have CNS malignancies. J Clin Oncol. 2005; 23:7152–7160. PMID: 16192599.
Article3. Kortmann RD, Kühl J, Timmermann B, et al. Postoperative-neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT '91. Int J Radiat Oncol Biol Phys. 2000; 46:269–279. PMID: 10661332.4. Taylor RE, Bailey CC, Robinson K, et al. Results of a randomized study of preradiation chemotherapy versus radiotherapy alone for nonmetastatic medulloblastoma: The International Society of Paediatric Oncology/United Kingdom Children's Cancer Study Group PNET-3 Study. J Clin Oncol. 2003; 21:1581–1591. PMID: 12697884.
Article5. Packer RJ, Goldwein J, Nicholson HS, et al. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: a Children's Cancer Group Study. J Clin Oncol. 1999; 17:2127–2136. PMID: 10561268.
Article6. Packer RJ, Gurney JG, Punyko JA, et al. Long-term neurologic and neurosensory sequelae in adult survivors of a childhood brain tumor: childhood cancer survivor study. J Clin Oncol. 2003; 21:3255–3261. PMID: 12947060.
Article7. Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2006; 24:5277–5282. PMID: 17088567.
Article8. Shuper A, Packer RJ, Vezina LG, Nicholson HS, Lafond D. 'Complicated migraine-like episodes' in children following cranial irradiation and chemotherapy. Neurology. 1995; 45:1837–1840. PMID: 7477978.
Article9. Thomas PR, Deutsch M, Kepner JL, et al. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol. 2000; 18:3004–3011. PMID: 10944134.
Article10. Verlooy J, Mosseri V, Bracard S, et al. Treatment of high risk medulloblastomas in children above the age of 3 years: a SFOP study. Eur J Cancer. 2006; 42:3004–3014. PMID: 16956759.
Article11. Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group Study. J Clin Oncol. 1998; 16:1723–1728. PMID: 9586884.
Article12. Sung KW, Lee SH, Yoo KH, et al. Tandem high-dose chemotherapy and autologous stem cell rescue in patients over 1 year of age with stage 4 neuroblastoma. Bone Marrow Transplant. 2007; 40:37–45. PMID: 17468771.
Article13. Matthay KK, Villablanca JG, Seeger RC, et al. Treatment of high-risk neuroblastoma with intensive chemotherapy, radiotherapy, autologous bone marrow transplantation, and 13-cis-retinoic acid. Children's Cancer Group. N Engl J Med. 1999; 341:1165–1173. PMID: 10519894.14. Strother D, Ashley D, Kellie SJ, et al. Feasibility of four consecutive high-dose chemotherapy cycles with stem-cell rescue for patients with newly diagnosed medulloblastoma or supratentorial primitive neuroectodermal tumor after craniospinal radiotherapy: results of a collaborative study. J Clin Oncol. 2001; 19:2696–2704. PMID: 11352962.
Article15. Sung KW, Yoo KH, Cho EJ, et al. High-dose chemotherapy and autologous stem cell rescue in children with newly diagnosed high-risk or relapsed medulloblastoma or supratentorial primitive neuroectodermal tumor. Pediatr Blood Cancer. 2007; 48:408–415. PMID: 17066462.
Article16. Gilbertson RJ. Medulloblastoma: signalling a change in treatment. Lancet Oncol. 2004; 5:209–218. PMID: 15050952.
Article17. Taylor RE, Bailey CC, Robinson KJ, et al. Outcome for patients with metastatic (M2-3) medulloblastoma treated with SIOP/UKCCSG PNET-3 chemotherapy. Eur J Cancer. 2005; 41:727–734. PMID: 15763649.
Article18. Bailey CC, Gnekow A, Wellek S, et al. Prospective randomised trial of chemotherapy given before radiotherapy in childhood medulloblastoma. International Society of Paediatric Oncology (SIOP) and the (German) Society of Paediatric Oncology (GPO): SIOP II. Med Pediatr Oncol. 1995; 25:166–178. PMID: 7623725.
Article19. Kortmann RD, Kühl J, Timmermann B, et al. Postoperative neoadjuvant chemotherapy before radiotherapy as compared to immediate radiotherapy followed by maintenance chemotherapy in the treatment of medulloblastoma in childhood: results of the German prospective randomized trial HIT '91. Int J Radiat Oncol Biol Phys. 2000; 46:269–279. PMID: 10661332.
Article20. Gajjar A, Chintagumpala M, Ashley D, et al. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006; 7:813–820. PMID: 17012043.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- High-dose chemotherapy with reduced-dose craniospinal radiotherapy in children with newly diagnosed high-risk brain tumor
- Thyroid dysfunction in patients with childhood-onset medulloblastoma or primitive neuroectodermal tumor
- Treatment of Supratentorial Primitive Neuroectodermal Tumors (PNETs) in Children
- A Case of Peripheral Primitive Neuroectodermal Tumor of the Vulva
- High-dose chemotherapy and autologous stem cell transplantation for pediatric brain tumors