Korean J Physiol Pharmacol.
2010 Dec;14(6):371-376. 10.4196/kjpp.2010.14.6.371.
Effects of Glycyrrhizae Radix on Repeated Restraint Stress-induced Neurochemical and Behavioral Responses
- Affiliations
-
- 1Department of Integrative Medicine and the Research Center of Behavioral Medicine, College of Medicine, The Catholic University of Korea, Seoul 137-701, Korea.
- 2Acupuncture and Meridian Science Research Center, Kyung Hee University, Seoul 130-701, Korea. ishim@khu.ac.kr
- 3Division of Brain Disease, Center for Biomedical Science, National Institute of Health, Seoul 122-701, Korea.
- KMID: 2071708
- DOI: http://doi.org/10.4196/kjpp.2010.14.6.371
Abstract
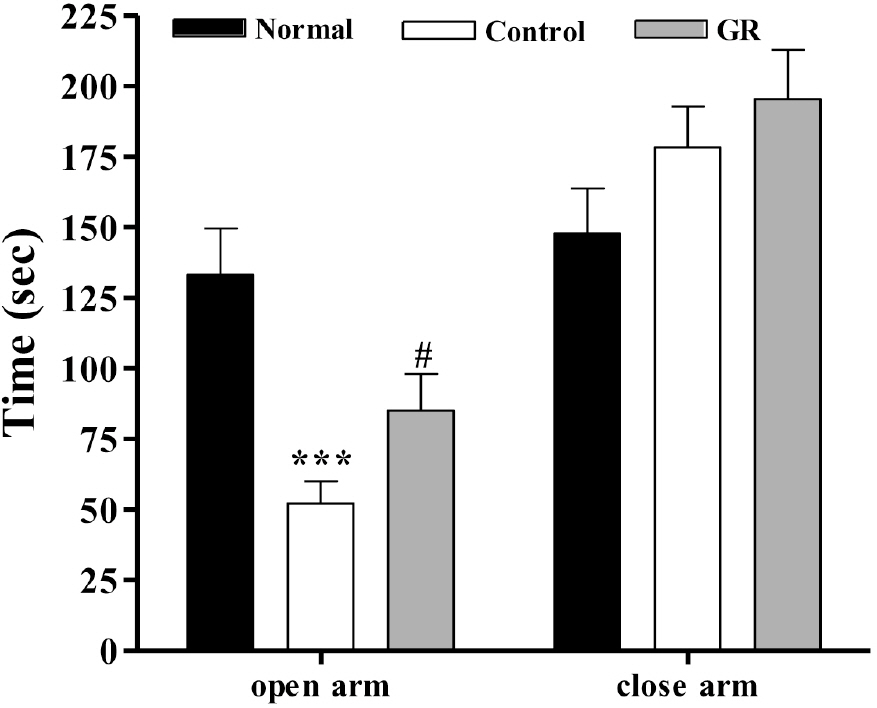
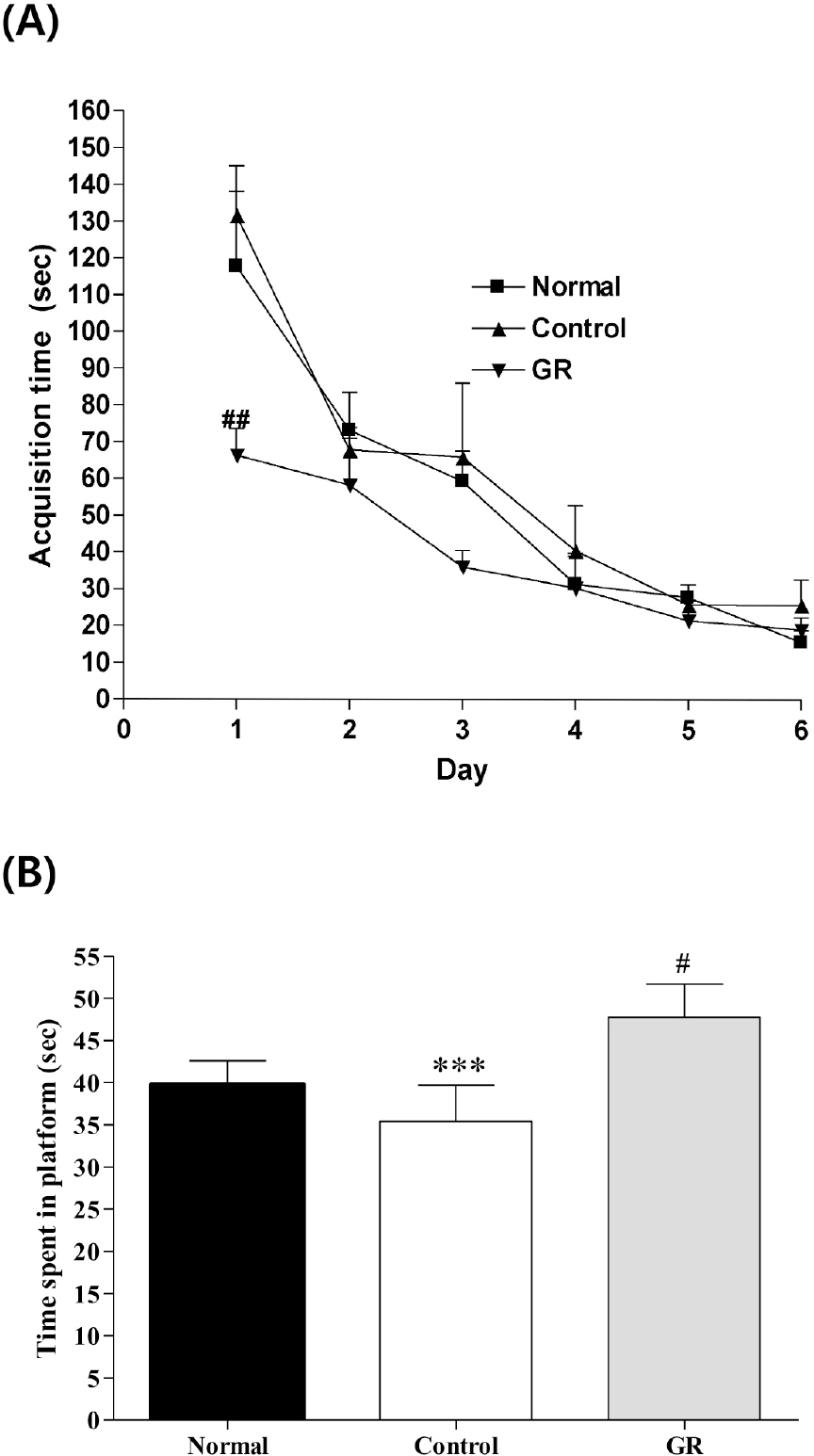
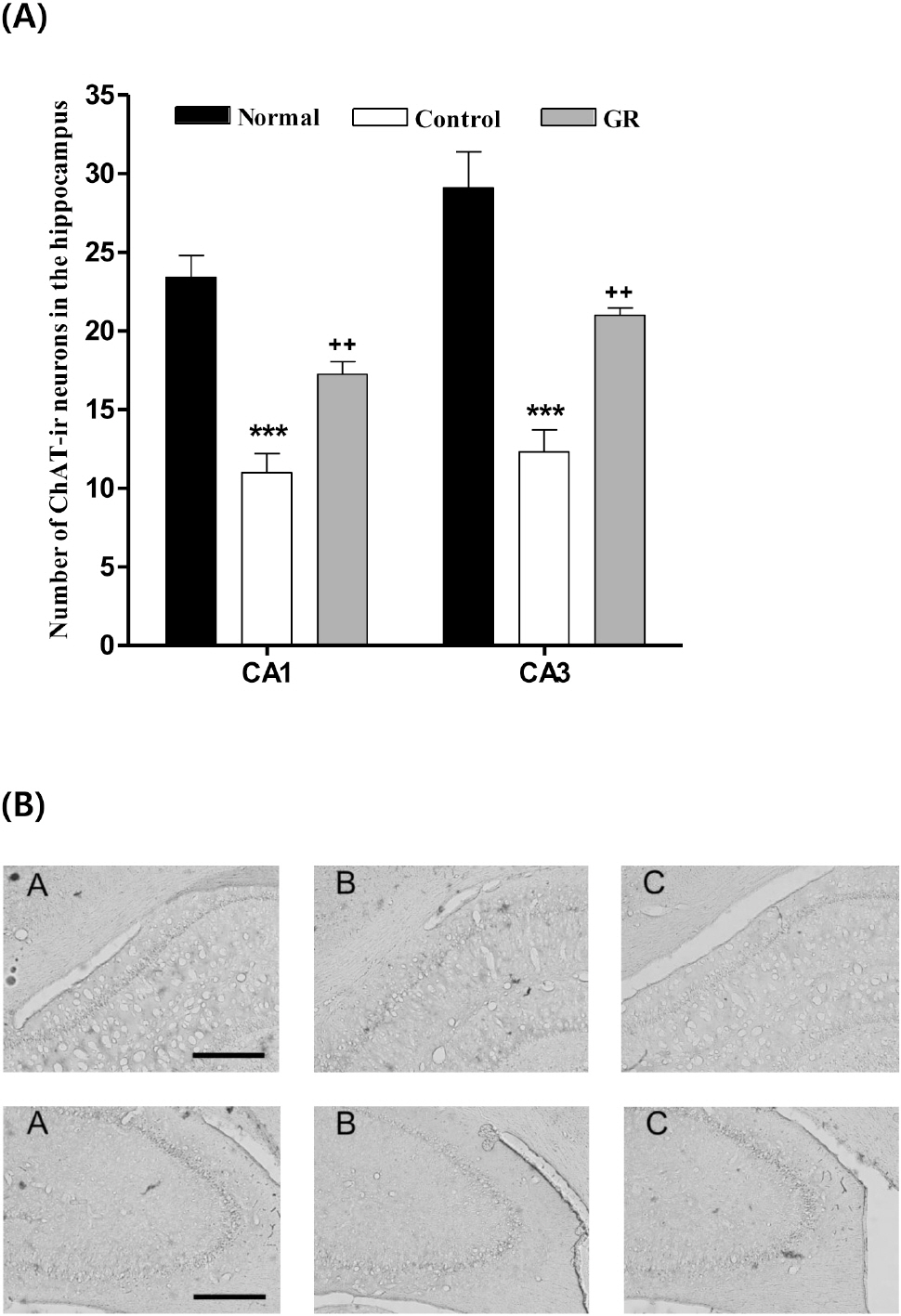
- Glycyrrhizae radix (GR) is an herbal medicine that is commonly used in the East Asia for treating a variety of diseases, including stomach disorders. The objective of the present study was to examine the anti-stress effects of GR on repeated stress-induced alterations of anxiety, learning and memory in rats. Restraint stress was administered for 14 days (2 h/day) to the rats in the Control and GR groups (400 mg/kg/day, PO). Starting on the eighth day, the rats were tested for spatial memory on the Morris water maze test (MW) and for anxiety on the elevated plus maze (EPM). We studied the changes of the expressions of cholineacetyl transferase (ChAT) and tyrosine hydroxylase (TH) in the locus coerleus (LC) using immunohistochemistry. The results showed that the rats treated with GR had significantly reduced stress-induced deficits on their learning and memory on the spatial memory tasks. In addition, the ChAT immunoreactivities were increased. Gor the EPM, treatment with GR increased the time spent in the open arms (p<0.001) as compared to that of the control group. Moreover, GR treatment also normalized the increases of the TH expression in the LC (p<0.001). In conclusion, administration of GR improved spatial learning and memory and reduced stress-induced anxiety. Thus, the present results suggest that GR has the potential to attenuate the behavioral and neurochemical impairments caused by stress.
MeSH Terms
Figure
Reference
-
References
1. Chrousos GP. Stressor, stress and neuroendocrinology intergration of the adaptive response. The 1997 Hans Selye Memorial lecture. Ann NY Acad Sci. 1998; 851:311–335.2. Pare WP. The effects of chronic environmental stress and stomach ulceration, adrenal function and consummatory behavior in the rat. J Psychol. 1964; 57:143–151.3. Luine V, Villegas M, Martinez C, McEwen BS. Repeated stress causes reversibal impairments of spatial memory performance. Brain Res. 1994; 639:167–170.4. File SE. The use of social interaction as a method for detecting anxiolytic activity of chlrodiazepoxide-like drugs. J Neurosci Methods. 1980; 2:219–238.5. Bisagno V, Grillo CA, Piroli GG, Giraldo P, MaEwen B, Luine VN. Chronic stress alters amphetamine effects on behavior and synaprophysin levels in female rats. Pharmacol Biochem Behav. 2004; 78:541–550.6. Bodnoff SR, Humphreys AG, Lehman JC, Diamond DM, Rose GM, Meaney MJ. Enduring effects of chronic corticoserone treatment on spatial learning, synaptic plasticity, and hippocampal neuropathology in young and mid-aged rats. J Neurosci. 1995; 15:61–69.7. Coburn-Litvak PS, Tata DA, Gorby HE, McCloskey DP, Richardson G, Anderson BJ. Chronic corticosterone affects brain weight, and mitochondrial, but nor glial volume fraction in hippocampal area CA3. Neuroscience. 2004; 124:429–438.8. Krugers HJ, Goltstein PM, van der Linden S, Joels M. Blockade of glucocorticoid receptors rapidly restores hippocampal CA1 synaptic plasticity after exposure to chronic stress. Eur J Neurosci. 2006; 23:3051–3055.
Article9. Mclay R, Klinski A. Changes in prescription habits with the introduction of generic fluoxetine. Mil Med. 2008; 173:100–104.
Article10. Sousa N, Almeida OF, Holsboer F, Paula-Barbosa MM, Madeira MD. Maintenance of hippocampal cell numbers in young and aged rats submitted to chronic unpredictable stress. Comparison with the effects of corticosterone treatment. Stress. 1998; 2:237–249.
Article11. Ader R, Cohen N. Psychoneuroimmunology: conditioning and stress. Annu Rev Psychol. 1993; 44:53–85.
Article12. McEwen BS, Stellar E. Stress and the individual:mechanisms leading to disease. Arch Intern Med. 1993; 153:2093–2101.13. Wang ZY, Nixon DW. Licorice and cancer. Nutr Cancer. 2001; 39:1–11.
Article14. Kim SC, Byun SH, Yang CH, Kim CY, Kim JW, Kim SG. Cytoprotective aeffects of Glycyrrhizae radix extract and its active component liquiritigenin against cadmium-induced toxicity (effects on bad translocation and PARP cleavage. Toxicology. 2004; 197:239–251.15. Kee CH. The pharmacology of Chinese herbs. 2nd ed.Boca Ranton: CRC press;1999. p. 363–367.16. Morris RG. A learning deficit related to age and β-amyloid plaques in a mouse model of Alzheimer's disease. Nature. 2000; 408:975–979.17. Paxinos G, Watson C, Pennisi M. Bregma, lamda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985; 13:139–143.18. Wang YT, Tan QR, Sun LL, Cao J, Dou KF, Xia B, Wang W. Possible therapeutic effect of a traditional chinese medicine, sinisan, on chronic restraint stress related disorders. Neurosci Lett. 2008; 449:215–219.
Article19. Oda Y. Choline acetyltransferase: the structurem distribution and pathologic changes in the central nervous system. Pathology International. 1999; 49:921–937.20. Giacobini E. Long term stabilizing effect of cholinesterase inhibitors in the therapy of Alzheimer's disease. J Neural Transm Suppl. 2002; 62:181–187.21. Gottesfeld Z, Kvetnansky R, Kopin IJ, Jacobowitz DM. Effects of repeated immobilization stress on glutamate decarboxylase and choline acetyltransferase in discrete brain regions. Brain Res. 1978; 152:374–378.22. Park HJ, Han SM, Yoon WJ, Kim KS, Shim I. The effect of Puerariae Flos on stress-induced deficits of learning and memory in ovariectomized female rats. Korean J Physiol Pharmacol. 2009; 13:85–89.23. Dinan TG, Aston-Jones G. Acute haloperidol increases impulse activity of brain noradrenergic neurons. Brain Res. 1984; 307:359–362.
Article24. Sabban EL. Catecholamines in stress: molecular mechanisms of gene expression. Endocr Regul. 2007; 41:61–73.25. Foote SL, Berridge CW, Adams LM, Pineda JA. Electrophysiological evidence for the involvement of the locus coeruleus in alerting, orienting, and attending. Prog Brain Res. 1991; 88:521–532.
Article26. Redmond DE Jr, Huang YH. The primate locus coerleus and effects of clonidine on opiate withdrawal. J Clin Psychiatry. 1982; 43:25–29.27. Melia KR, Rasmussen RZ, Terwillinger JW, Haycook EJ, Nestler RS. Coordinate regulation of the cyclic AMP system with firing rate and expression of tyrosine hydroxylase the rat locus coeruleus: Effects of chronic stress and drug treatment. J Neurochem. 1992; 58:494–502.28. Valentino RJ, Foote SL. Corticotropin-releasing factor distrups sensory responses of brain noradrenergic neurons. Neuroendocrinology. 1987; 45:28–36.29. Valentino RJ, Foote SL, Page ME. The locus coeruleus as a site for integrating corticotrophin-releasing factor and noradrenergic mediation of stress responses. Ann N Y Acad Sci. 1993; 697:173–188.30. Ma S, Mifflin SW, Cunningham JT, Mortilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitaryadrenal stress reactivity and Fos induction in the rat locus coerleus in response to subsequent immobilization stress. Neuroscience. 2008; 154:1639–1647.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Astragalus Membranaceus on Repeated Restraint Stress-induced Biochemical and Behavioral Responses
- Chronic Administration of Baicalein Decreases Depression-Like Behavior Induced by Repeated Restraint Stress in Rats
- Protective effect of an ethanol extract mixture of Aralia elata, Chaenomeles sinensis fruit, and Glycyrrhizae radix against cerebral ischemiareperfusion injury in rats and excitotoxic and oxidative neuronal damage
- Effects of Prenatal Stress and Restraint Stress on Amygdala Complex of the Rat: II. Effects on the Astrocytic Cell Processes
- Effects of repeated restraint stress on platelet endothelial cell adhesion molecule-1 immunoreactivity and protein levels in the gerbil hippocampus after transient cerebral ischemia