Korean J Physiol Pharmacol.
2009 Jun;13(3):221-227. 10.4196/kjpp.2009.13.3.221.
Accurate Representation of Light-intensity Information by the Neural Activities of Independently Firing Retinal Ganglion Cells
- Affiliations
-
- 1Department of Biomedical Engineering, College of Health Science, Yonsei University, Wonju 220-710, Korea. khkim0604@yonsei.ac.kr
- 2Department of Physiology, Chungbuk National University School of Medicine, Cheongju 361-763, Korea.
- 3Nano Artificial Vision Research Center, Seoul National University Hospital, Seoul 110-744, Korea.
- KMID: 2071675
- DOI: http://doi.org/10.4196/kjpp.2009.13.3.221
Abstract
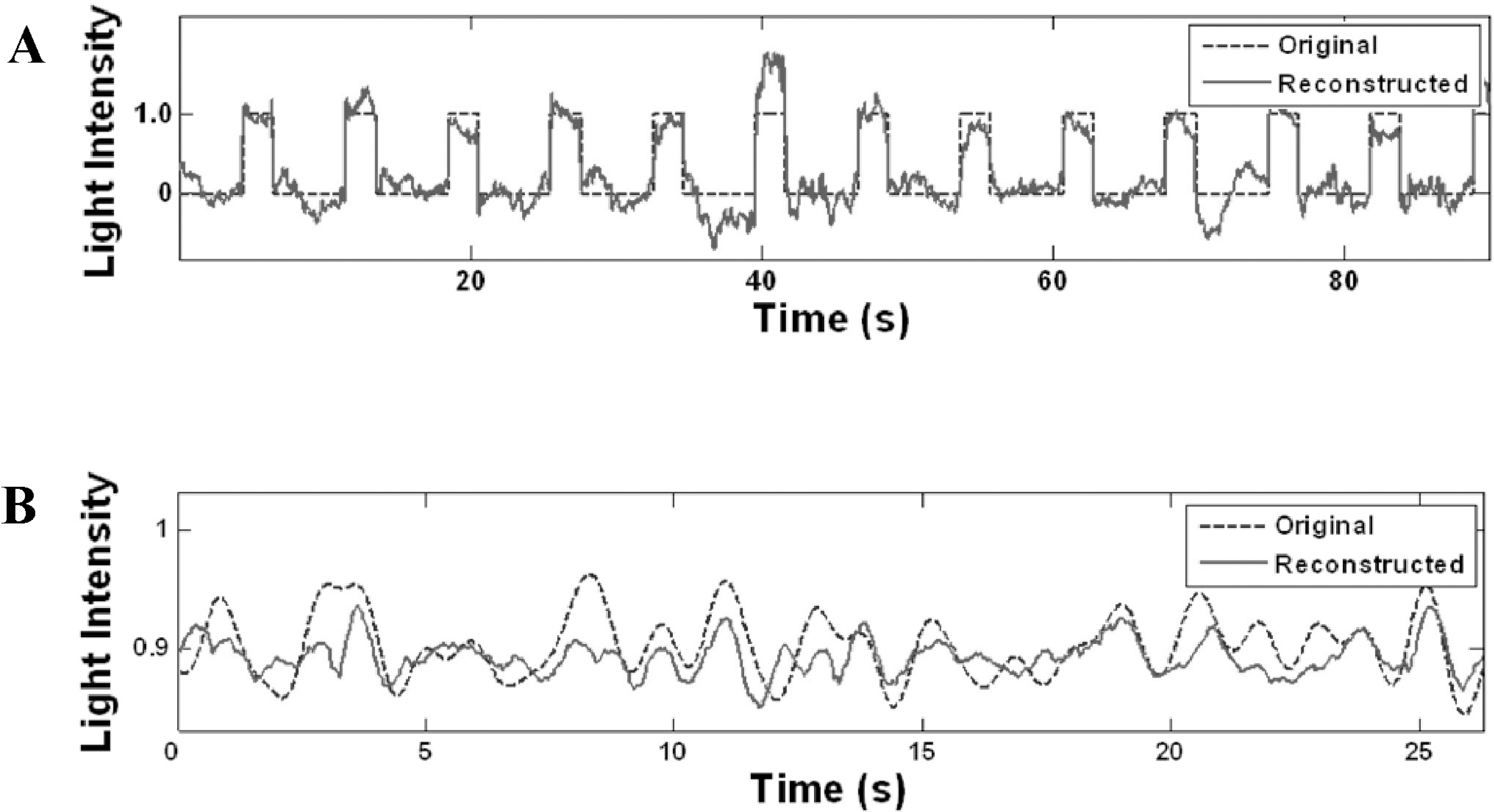
- For successful restoration of visual function by a visual neural prosthesis such as retinal implant, electrical stimulation should evoke neural responses so that the information on visual input is properly represented. A stimulation strategy, which means a method for generating stimulation waveforms based on visual input, should be developed for this purpose. We proposed to use the decoding of visual input from retinal ganglion cell (RGC) responses for the evaluation of stimulus encoding strategy. This is based on the assumption that reliable encoding of visual information in RGC responses is required to enable successful visual perception. The main purpose of this study was to determine the influence of inter-dependence among stimulated RGCs activities on decoding accuracy. Light intensity variations were decoded from multiunit RGC spike trains using an optimal linear filter. More accurate decoding was possible when different types of RGCs were used together as input. Decoding accuracy was enhanced with independently firing RGCs compared to synchronously firing RGCs. This implies that stimulation of independently-firing RGCs and RGCs of different types may be beneficial for visual function restoration by retinal prosthesis.
Keyword
MeSH Terms
Figure
Reference
-
Brown EN., Kass RE., Mitra PP. Multiple neural spike train data analysis: state-of-the-art and future challenges. Nat Neurosci. 7:456–461. 2004.
ArticleBrown SP., He S., Masland RH. Receptive field microstructure and dendritic geometry of retinal ganglion cells. Neuron. 27:371–383. 2000.
ArticleCho HS., Jin GH., Goo YS. Characterization of rabbit retinal ganglion cells with multichannel recording. Korean J Med Phys. 15:228–236. 2004.Field GD., Chichilnisky EJ. Information processing in the primate retina: circuitry and coding. Annu Rev Neurosci. 30:1–30. 2007.
ArticleFried SI., Hsueh HA., Werblin FS. A method for generating precise temporal patterns of retinal spiking using prosthetic stimulation. J Neurophysiol. 95:970–978. 2006.
ArticleHumayun MS., Weiland JD., Fujii GY., Greenberg R., Williamson R., Little J., Mech B., Cimmarusti V., Boemel GV., Dagnelie G., de Juan E. Visual perception in a blind subject with a chronic microelectronic retinal prosthesis. Vision Res. 43:2573–2581. 2003.
ArticleKim KH., Kim SS., Kim SJ. Superiority of nonlinear mapping in decoding multiple single-unit neuronal spike trains: a simulation study. J Neurosci Methods. 150:202–211. 2006.
ArticleMajji AB., Humayun MS., Weiland JD., Suzuki S., D'Anna SA., de Juan E. Long-term histological and electrophysiological results of an inactive epiretinal electrode array implantation in dogs. Invest Ophthalmol Vis Sci. 40:2073–2081. 1999.Meister M., Lagnado L., Baylor DA. Concerted signaling by retinal ganglion cells. Science. 270:1207–1210. 1995.
ArticleMerabet LB., Rizzo JF., Amedi A., Somers DC., Pascual-Leone A. What blindness can tell us about seeing again: merging neuro-plasticity and neuroprosthesis. Nat Rev Neurosci. 6:71–77. 2005.Nicolelis MAL. Methods for Neural Ensemble recording. CRC Press, Florida. 1998.Rizzo JF., Wyatt J., Loewenstein J., Kelly S., Shire D. Methods and perceptual thresholds for short-term electrical stimulation of human retina with microelectrode arrays. Invest Ophthalmol Vis Sci. 44:5355–5361. 2003.
ArticleRyu SB., Kim DH., Ye JH., Kim KH., Goo YS. Estimation of visual stimulus intensity from retinal ganglion cell spike trains using optimal linear filter. J Biomed Eng Res. 28:212–217. 2007.Santos A., Humayun MS., de Juan E., Greenburg RJ., Marsh MJ., Klock IB., Milam AM. Preservation of the inner retina in retinitis pigmentosa:. a morphometric analysis. Arch Ophthal. 115:511–515. 1997.
ArticleSekirnjak C., Hottowy P., Sher A., Dabrowski W., Litke AM., Chichilnisky EJ. Electrical stimulation of mammalian retinal ganglion cells with multielectrode array. J Neurophysiol. 95:3311–3327. 2006.Stett A., Barth W., Weiss S., Haemmerle H., Zrenner E. Electrical multisite stimulation of the isolated chicken retina. Vision Res. 40:1785–1795. 2000.
ArticleWarland DK., Reinagel P., Meister M. Decoding visual information from a population of retinal ganglion cells. J Neurophysiol. 78:2336–2350. 1997.
ArticleWessberg J., Stambaugh CR., Kralik JD., Beck PD., Laubach M., Chapin JK., Kim J., Biggs SJ., Srinivasan MA., Nicolelis MA. Real-time prediction of hand trajectory by ensembles of cortical neurons in primates. Nature. 408:361–365. 2000.
ArticleYe JH., Ryu SB., Kim KH., Goo YS. Functional connectivity map of retinal ganglion cells for retinal prosthesis. Korean J Physiol Pharmacol. 12:307–314. 2008.
ArticleZrenner E. Will retinal implants restore vision? Science. 295:1022–1025. 2002.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Amplitude Modulation-based Electrical Stimulation for Encoding Multipixel Spatiotemporal Visual Information in Retinal Neural Activities
- NF-kappa B activation following optic nerve transection
- Electrically-evoked Neural Activities of rd1 Mice Retinal Ganglion Cells by Repetitive Pulse Stimulation
- Characteristics of Light-evoked Retinal Ganglion Cell Activity with Postnatal Maturation in SD Rat
- Synchrony of Spontaneous Burst Firing between Retinal Ganglion Cells Across Species