J Vet Sci.
2014 Dec;15(4):459-464. 10.4142/jvs.2014.15.4.459.
Intragastric gavage with denatonium benzoate acutely induces neuronal activation in the solitary tract nucleus via the vagal afferent pathway
- Affiliations
-
- 1Department of Anatomy and Cell Biology, College of Veterinary Medicine, and Research Institute for Veterinary Science, Seoul National University, Seoul 151-742, Korea. vetmed2@snu.ac.kr
- 2Department of Anatomy, College of Veterinary Medicine, Kangwon National University, Chuncheon 200-701, Korea.
- 3Division of Metabolism and Functionality Research, Korea Food Research Institute, Sungnam 463-746, Korea.
- KMID: 2070231
- DOI: http://doi.org/10.4142/jvs.2014.15.4.459
Abstract
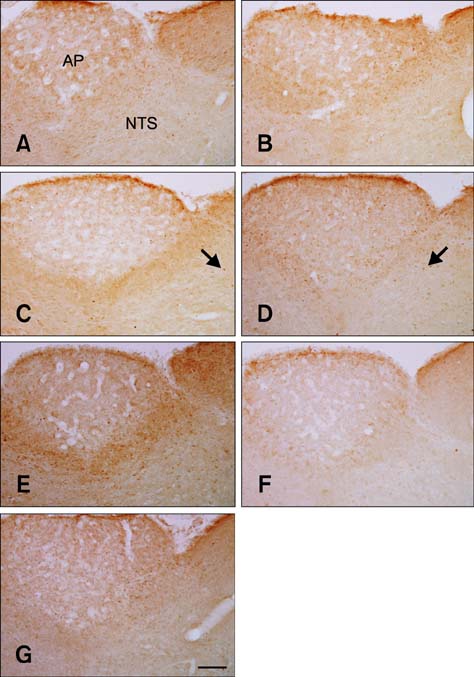
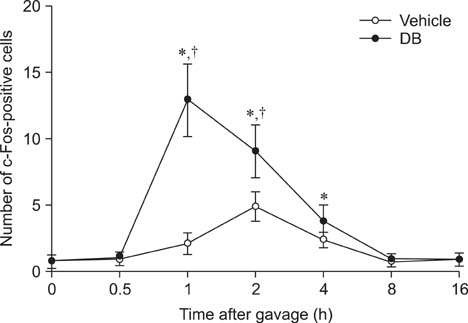
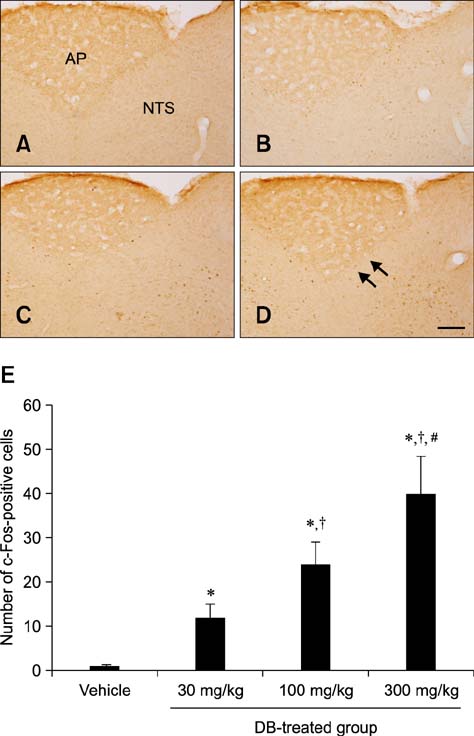
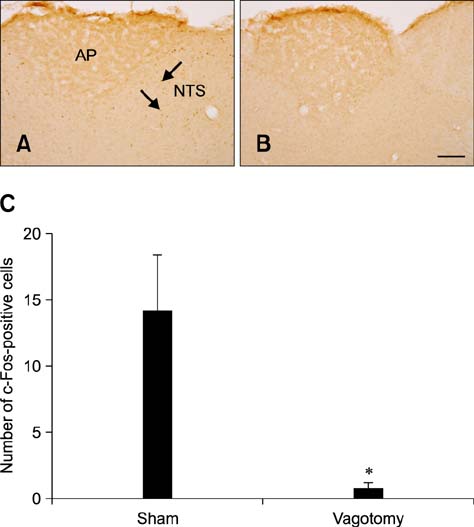
- Natural toxic substances have a bitter taste and their ingestion sends signals to the brain leading to aversive oral sensations. In the present study, we investigated chronological changes in c-Fos immunoreactivity in the nucleus tractus solitarius (NTS) to study the bitter taste reaction time of neurons in the NTS. Equal volumes (0.5 mL) of denatonium benzoate (DB), a bitter tastant, or its vehicle (distilled water) were administered to rats intragastrically. The rats were sacrificed at 0, 0.5, 1, 2, 4, 8, or 16 h after treatment. In the vehicle-treated group, the number of c-Fos-positive nuclei started to increase 0.5 h after treatment and peaked 2 h after gavage. In contrast, the number of c-Fos-positive nuclei in the DB-treated group significantly increased 1 h after gavage. Thereafter, the number of c-Fos immunoreactive nuclei decreased over time. The number of c-Fos immunoreactive nuclei in the NTS was also increased in a dose-dependent manner 1 h after gavage. Subdiaphragmatic vagotomy significantly decreased DB-induced neuronal activation in the NTS. These results suggest that intragastric DB increases neuronal c-Fos expression in the NTS 1 h after gavage and this effect is mediated by vagal afferent fibers.
MeSH Terms
-
Adjuvants, Immunologic/pharmacology
Afferent Pathways/physiology
Animals
Injections/veterinary
Ligands
Male
Proto-Oncogene Proteins c-fos/*metabolism
Quaternary Ammonium Compounds/*pharmacology
Rats
Rats, Sprague-Dawley
Receptors, G-Protein-Coupled/*metabolism
Solitary Nucleus/*physiology
Vagus Nerve/*drug effects/*physiology
Adjuvants, Immunologic
Ligands
Proto-Oncogene Proteins c-fos
Quaternary Ammonium Compounds
Receptors, G-Protein-Coupled
Figure
Reference
-
1. Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988; 242:1047–1050.
Article2. Dragunow M, Faull R. The use of c-fos as a metabolic marker in neuronal pathway tracing. J Neurosci Methods. 1989; 29:261–265.3. Furudono Y, Ando C, Kobashi M, Yamamoto C, Yamamoto T. The role of orexigenic neuropeptides in the ingestion of sweet-tasting substances in rats. Chem Senses. 2005; 30:Suppl 1. i186–il87.
Article4. Grundy D. Sensory signals from the gastrointestinal tract. J Pediatr Gastroenterol Nutr. 2005; 41:Suppl 1. S7–S9.
Article5. Gschossmann JM, Mayer EA, Miller JC, Raybould HE. Subdiaphragmatic vagal afferent innervation in activation of an opioidergic antinociceptive system in response to colorectal distension in rats. Neurogastroenterol Motil. 2002; 14:403–408.
Article6. Hao S, Dulake M, Espero E, Sternini C, Raybould HE, Rinaman L. Central Fos expression and conditioned flavor avoidance in rats following intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2009; 296:R528–R536.
Article7. Hao S, Sternini C, Raybould HE. Role of CCK1 and Y2 receptors in activation of hindbrain neurons induced by intragastric administration of bitter taste receptor ligands. Am J Physiol Regul Integr Comp Physiol. 2008; 294:R33–R38.
Article8. Harrer MI, Travers SP. Topographic organization of Fos-like immunoreactivity in the rostral nucleus of the solitary tract evoked by gustatory stimulation with sucrose and quinine. Brain Res. 1996; 711:125–137.
Article9. Höfer D, Püschel B, Drenckhahn D. Taste receptor-like cells in the rat gut identified by expression of α-gustducin. Proc Natl Acad Sci U S A. 1996; 93:6631–6634.
Article10. Houpt TA, Berlin R, Smith GP. Subdiaphragmatic vagotomy does not attenuate c-Fos induction in the nucleus of the solitary tract after conditioned taste aversion expression. Brain Res. 1997; 747:85–91.
Article11. Janssen S, Laermans J, Verhulst PJ, Thijs T, Tack J, Depoortere I. Bitter taste receptors and α-gustducin regulate the secretin of ghrelin with functional effects on food intake and gastric emptying. Proc Natl Acad Sci USA. 2011; 108:2094–2099.
Article12. King CT, Travers SP, Rowland NE, Garcea M, Spector AC. Glossopharyngeal nerve transection eliminates quinine-stimulated fos-like immunoreactivity in the nucleus of the solitary tract: implications for a functional topography of gustatory nerve input in rats. J Neurosci. 1999; 19:3107–3121.
Article13. Mueller KL, Hoon MA, Erlenbach I, Chandrashekar J, Zuker CS, Ryba NJ. The receptors and coding logic for bitter taste. Nature. 2005; 434:225–229.
Article14. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th ed. Amsterdam: Academic Press;2007. p. 139–144.15. Rozengurt N, Wu SV, Chen MC, Huang C, Sternini C, Rozengurt E. Colocalization of the α-subunit of gustducin with PYY and GLP-1 in L cells of human colon. Am J Physiol Gastrointest Liver Physiol. 2006; 291:G792–G802.
Article16. Sternini C. Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G457–G461.
Article17. Sutherland K, Young RL, Cooper NJ, Horowitz M, Blackshaw LA. Phenotypic characterization of taste cells of the mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007; 292:G1420–G1428.
Article18. Travers JB, Urbanek K, Grill HJ. Fos-like immunoreactivity in the brain stem following oral quinine stimulation in decerebrate rats. Am J Physiol. 1999; 277:R384–R394.19. Uneyama H, Tanaka T, Torii K. Gut nutrient sensing by the abdominal vagus. Nihon Yakurigaku Zasshi. 2004; 124:210–218.
Article20. Wu SV, Chen MC, Rozengurt E. Genomic organization, expression, and function of bitter taste receptors (T2R) in mouse and rat. Physiol Genomics. 2005; 22:139–149.
Article21. Wu SV, Rozengurt N, Yang M, Young SH, Sinnett-Smith J, Rozengurt E. Expression of bitter taste receptors of the T2R family in the gastrointestinal tract and enteroendocrine STC-1 cells. Proc Natl Acad Sci U S A. 2002; 99:2392–2397.
Article22. Yamamoto T, Sawa K. Comparison of c-Fos-like immunoreactivity in the brainstem following intraoral and intragastric infusions of chemical solutions in rats. Brain Res. 2000; 866:144–151.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Centrifugal Influence on Gustatory Neurons in the Nucleus of the Solitary Tract
- Characteristics of Vagal Esophageal Tension-Sensitive Afferent Fibers in the Cat
- Ginger and Its Pungent Constituents Non-Competitively Inhibit Serotonin Currents on Visceral Afferent Neurons
- c-Fos-like Immunoreactivity in the nucleus of the solitary tract following taste stimulation of the contralateral side of the tongue
- Subdiaphragmatic vagotomy induces NADPH diaphorase in the rat dorsal motor nucleus of the vagus