Yonsei Med J.
2014 Nov;55(6):1664-1671. 10.3349/ymj.2014.55.6.1664.
ATP-Based Chemotherapy Response Assay in Primary or Recurrent Ovarian and Peritoneal Cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Seoul National University College of Medicine, Seoul, Korea.
- 2Institute of Women's Life Medical Science, Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Yonsei University College of Medicine, Seoul, Korea. shkim70@yuhs.ac
- 3Department of Obstetrics and Gynecology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- KMID: 2070217
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1664
Abstract
- PURPOSE
To investigate chemosensitivity with an adenosine triphosphate-based chemotherapy response assay in patients with epithelial ovarian or peritoneal cancer according to tumor histology, grade, and disease status.
MATERIALS AND METHODS
One hundred specimens were collected during primary or secondary debulking from 67 patients with primary ovarian cancer, 24 patients with recurrent ovarian cancer, 5 patients with primary peritoneal cancer, and 4 patients with recurrent peritoneal cancer; samples were collected between August 2006 and June 2009. Tumor cells were isolated and cultured for 48 hours in media containing chemotherapy. The chemosensitivity index (CI) was calculated as 300 minus the sum of the cell death rate at 0.2x, 1x, and 5x drug concentrations, and the CI values were compared.
RESULTS
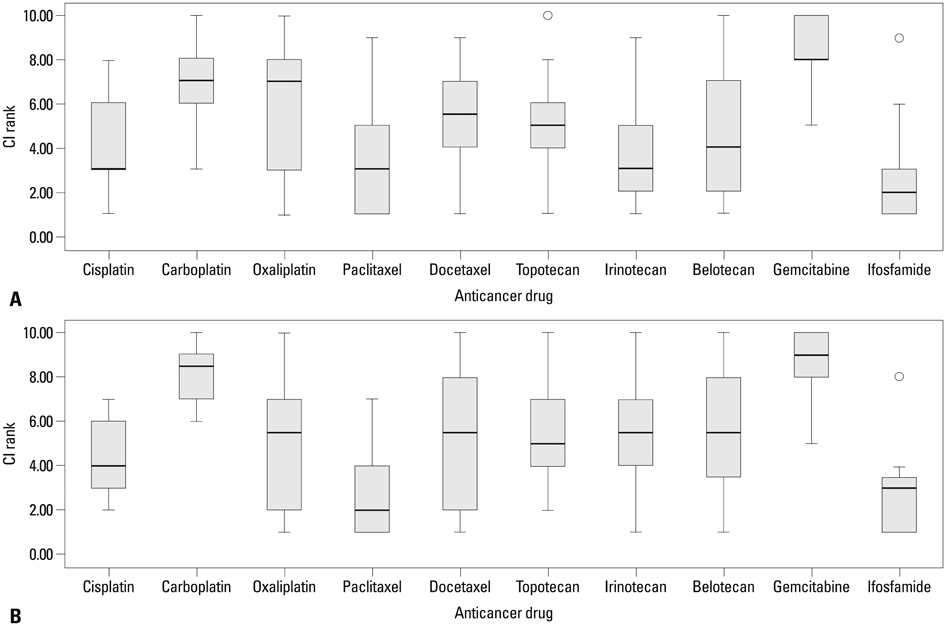
CI values were obtained from 93 of 100 patients. The most active agents against primary disease were ifosfamide and paclitaxel. For primary serous adenocarcinoma, paclitaxel and irinotecan were the most active, followed by ifosfamide. For clear cell carcinoma, ifosfamide was the most active, followed by paclitaxel and irinotecan. Although not statistically significant, the CIs of cisplatin, carboplatin, paclitaxel, and docetaxel decreased as tumor grade increased. In 14 cases of recurrent disease, paclitaxel was the most active, followed by ifosfamide and cisplatin.
CONCLUSION
Ifosfamide and paclitaxel were the most active drugs for primary and recurrent disease. Therefore, we recommend further clinical studies to confirm the efficacy of paclitaxel, ifosfamide, and cisplatin combination chemotherapy for recurrent and primary ovarian cancer.
MeSH Terms
-
Adenocarcinoma, Clear Cell/*drug therapy/metabolism/pathology
Adenosine Triphosphate/*metabolism
Adult
Aged
Antineoplastic Combined Chemotherapy Protocols/*therapeutic use
Camptothecin/administration & dosage/analogs & derivatives
Carboplatin/therapeutic use
Cisplatin/administration & dosage
Drug Resistance, Neoplasm
Drug Screening Assays, Antitumor/methods
Female
Humans
Ifosfamide/administration & dosage
Middle Aged
Neoplasm Recurrence, Local/*drug therapy
Neoplasms, Glandular and Epithelial/*drug therapy/metabolism/pathology
Ovarian Neoplasms/*drug therapy/metabolism/pathology
Paclitaxel/therapeutic use
Peritoneal Neoplasms/*drug therapy/metabolism/pathology
Predictive Value of Tests
Sensitivity and Specificity
Taxoids/administration & dosage
Adenosine Triphosphate
Camptothecin
Carboplatin
Cisplatin
Ifosfamide
Paclitaxel
Taxoids
Figure
Reference
-
1. Cree IA, Kurbacher CM. Individualizing chemotherapy for solid tumors--is there any alternative? Anticancer Drugs. 1997; 8:541–548.2. Kern DH, Drogemuller CR, Kennedy MC, Hildebrand-Zanki SU, Tanigawa N, Sondak VK. Development of a miniaturized, improved nucleic acid precursor incorporation assay for chemosensitivity testing of human solid tumors. Cancer Res. 1985; 45(11 Pt 1):5436–5441.3. Bird MC, Bosanquet AG, Forskitt S, Gilby ED. Semi-micro adaptation of a 4-day differential staining cytotoxicity (DiSC) assay for determining the in-vitro chemosensitivity of haematological malignancies. Leuk Res. 1986; 10:445–449.
Article4. Pieters R, Loonen AH, Huismans DR, Broekema GJ, Dirven MW, Heyenbrok MW, et al. In vitro drug sensitivity of cells from children with leukemia using the MTT assay with improved culture conditions. Blood. 1990; 76:2327–2336.
Article5. Hamburger AW, Salmon SE. Primary bioassay of human tumor stem cells. Science. 1977; 197:461–463.
Article6. Andreotti PE, Cree IA, Kurbacher CM, Hartmann DM, Linder D, Harel G, et al. Chemosensitivity testing of human tumors using a microplate adenosine triphosphate luminescence assay: clinical correlation for cisplatin resistance of ovarian carcinoma. Cancer Res. 1995; 55:5276–5282.7. Sevin BU, Peng ZL, Perras JP, Ganjei P, Penalver M, Averette HE. Application of an ATP-bioluminescence assay in human tumor chemosensitivity testing. Gynecol Oncol. 1988; 31:191–204.
Article8. O'Meara AT, Sevin BU. Predictive value of the ATP chemosensitivity assay in epithelial ovarian cancer. Gynecol Oncol. 2001; 83:334–342.9. Kim HA, Yom CK, Moon BI, Choe KJ, Sung SH, Han WS, et al. The use of an in vitro adenosine triphosphate-based chemotherapy response assay to predict chemotherapeutic response in breast cancer. Breast. 2008; 17:19–26.
Article10. Moon YW, Choi SH, Kim YT, Sohn JH, Chang J, Kim SK, et al. Adenosine triphosphate-based chemotherapy response assay (ATP-CRA)-guided platinum-based 2-drug chemotherapy for unresectable nonsmall-cell lung cancer. Cancer. 2007; 109:1829–1835.
Article11. Han SS, Choi SH, Lee YK, Kim JW, Park NH, Song YS, et al. Predictive value of individualized tumor response testing by ATP-based chemotherapy response assay in ovarian cancer. Cancer Invest. 2008; 26:426–430.
Article12. Kang SM, Park MS, Chang J, Kim SK, Kim H, Shin DH, et al. A feasibility study of adenosine triphosphate-based chemotherapy response assay (ATP-CRA) as a chemosensitivity test for lung cancer. Cancer Res Treat. 2005; 37:223–227.
Article13. Iinuma H, Okinaga K, Adachi M, Suda K, Sekine T, Sakagawa K, et al. Detection of tumor cells in blood using CD45 magnetic cell separation followed by nested mutant allele-specific amplification of p53 and K-ras genes in patients with colorectal cancer. Int J Cancer. 2000; 89:337–344.
Article14. Weisenthal LM, Dill PL, Finklestein JZ, Duarte TE, Baker JA, Moran EM. Laboratory detection of primary and acquired drug resistance in human lymphatic neoplasms. Cancer Treat Rep. 1986; 70:1283–1295.15. Bird MC, Bosanquet AG, Gilby ED. In vitro determination of tumour chemosensitivity in haematological malignancies. Hematol Oncol. 1985; 3:1–10.
Article16. Zylberberg B, Dormont D, Madelenat P, Daraï E. First-line intraperitoneal cisplatin-paclitaxel and intravenous ifosfamide in Stage IIIc ovarian epithelial cancer. Eur J Gynaecol Oncol. 2004; 25:327–332.17. Fruscio R, Colombo N, Lissoni AA, Garbi A, Fossati R, Ieda' N, et al. A phase II randomised clinical trial comparing cisplatin, paclitaxel and ifosfamide with cisplatin, paclitaxel and epirubicin in newly diagnosed advanced epithelial ovarian cancer: long-term survival analysis. Br J Cancer. 2008; 98:720–727.
Article18. Papadimitriou CA, Kouroussis C, Moulopoulos LA, Vlahos G, Rodolakis A, Kiamouris C, et al. Ifosfamide, paclitaxel and cisplatin first-line chemotherapy in advanced, suboptimally debulked epithelial ovarian cancer. Cancer. 2001; 92:1856–1863.
Article19. Polyzos A, Kosmas C, Tsavaris N, Toufexi H, Lagadas A, Gogas H, et al. Paclitaxel-ifosfamide-cisplatin as salvage chemotherapy in ovarian cancer patients pretreated with platinum compounds and paclitaxel. Anticancer Res. 2007; 27:1645–1651.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of Chemotherapy Sensitivity and Resistance Assays in Ovarian Cancer
- Chemotherapy for ovarian cancer
- Complete Remission of Unresectable Colon Cancer after Preoperative Chemotherapy Selected by Adenosine Triphosphate-Based Chemotherapy Response Assay
- Amylase-Producing Primary Peritoneal Carcinoma
- Long-term survival after intraperitoneal chemotherapy with paclitaxel-cisplatin for recurrent primary peritoneal cancer resistant to multiple lines of intravenous chemotherapy