Yonsei Med J.
2014 Nov;55(6):1617-1623. 10.3349/ymj.2014.55.6.1617.
Problems Associated with Alloplastic Materials in Rhinoplasty
- Affiliations
-
- 1Nose Aesthetic Plastic Surgery Clinic, Busan, Korea.
- 2I & Co Aesthetic Plastic Surgery Clinic, Busan, Korea.
- 3Department of Plastic and Reconstructive Surgery, Dong-A University School of Medicine, Busan, Korea. pokdungi@dau.ac.kr
- KMID: 2070211
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1617
Abstract
- PURPOSE
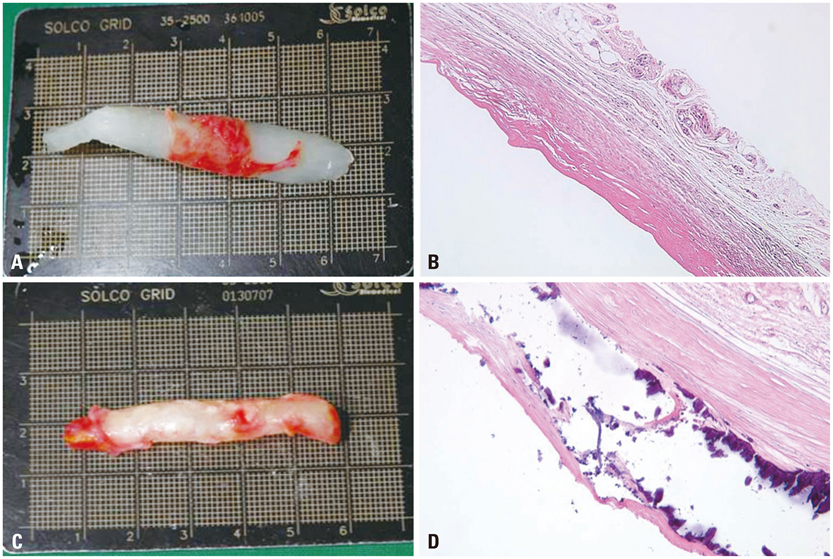
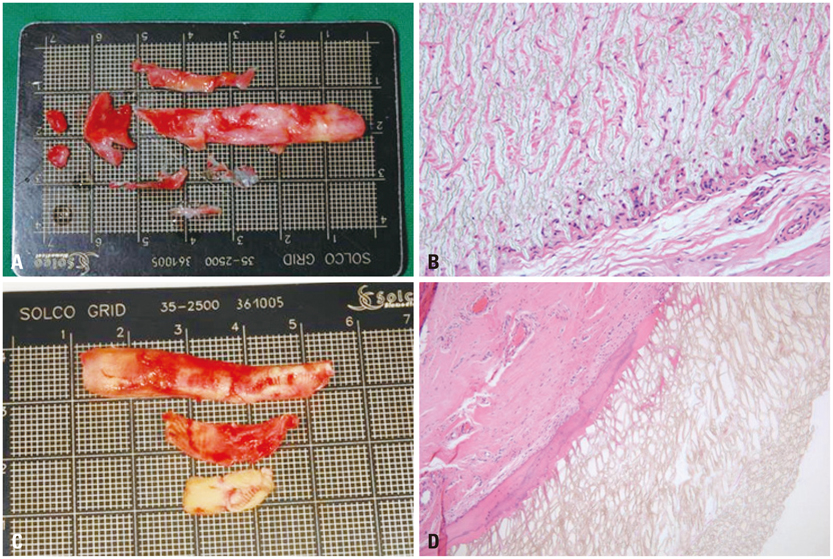
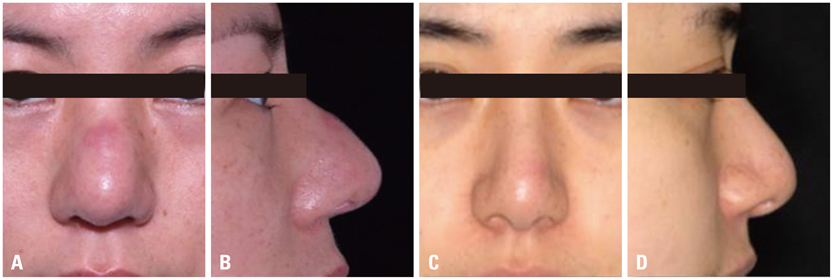
Augmentation rhinoplasty using alloplastic materials is a relatively common procedure among Asians. Silicon, expanded polytetrafluoroethylene (Gore-tex(R)), and porous high density polyethylene (Medpor(R)) are most frequently used materials. This study was conducted to analyze revisional rhinoplasty cases with alloplastic materials, and to investigate the usage of alloplastic materials and their complications. We also reviewed complications caused by various materials used in plastic surgery while operating rhinoplasty.
MATERIALS AND METHODS
We report 581 cases of complications rhinoplasty with alloplastic implants and review of the literature available to offer plastic surgeons an overview on alloplastic implant-related complications.
RESULTS
Among a total 581 revisional rhinoplasty cases reviewed, the alloplastic materials used were silicone implants in 376, Gore-tex(R) in 183, and Medpor(R) in 22 cases. Revision cases and complications differed according to each alloplastic implant.
CONCLUSION
Optimal alloplastic implants should be used in nasal structure by taking into account the properties of the materials for the goal of minimizing their complications and revision rates. A thorough understanding of the mechanism involved in alloplastic material interaction and wound healing is the top priority in successfully overcoming alloplastic-related complications.
Keyword
MeSH Terms
-
Asian Continental Ancestry Group
*Biocompatible Materials/adverse effects
Humans
Polyethylene
Polyethylenes
*Polytetrafluoroethylene
Postoperative Complications
Prosthesis Implantation/*methods
Rhinoplasty/*methods
Silicones
Treatment Outcome
Biocompatible Materials
Polyethylene
Polyethylenes
Polytetrafluoroethylene
Silicones
Figure
Cited by 3 articles
-
Maxillofacial reconstruction with Medpor porous polyethylene implant: a case series study
Mansour Khorasani, Pejman Janbaz, Farshid Rayati
J Korean Assoc Oral Maxillofac Surg. 2018;44(3):128-135. doi: 10.5125/jkaoms.2018.44.3.128.Augmentation Rhinoplasty with Expanded Polytetrafluoroethylene: A Ten-Year Experience for Safety
Dongmin Shin, Sihyung Kang, Yong Gi Jung
Korean J Otorhinolaryngol-Head Neck Surg. 2019;62(12):706-711. doi: 10.3342/kjorl-hns.2019.00346.Radiologic Findings of Complicated Alloplastic Implants in the Nasal Dorsum
Sung Hee Kim, Ji Won Kim, Yong Ju Jang
Clin Exp Otorhinolaryngol. 2021;14(3):321-327. doi: 10.21053/ceo.2020.01725.
Reference
-
1. Deva AK, Merten S, Chang L. Silicone in nasal augmentation rhinoplasty: a decade of clinical experience. Plast Reconstr Surg. 1998; 102:1230–1237.
Article2. Zeng Y, Wu W, Yu H, Yang J, Chen G. Silicone implant in augmentation rhinoplasty. Ann Plast Surg. 2002; 49:495–499.
Article3. Moon KM, Cho G, Sung HM, Jung MS, Tak KS, Jung SW, et al. Nasal anthropometry on facial computed tomography scans for rhinoplasty in Koreans. Arch Plast Surg. 2013; 40:610–615.
Article4. Tham C, Lai YL, Weng CJ, Chen YR. Silicone augmentation rhinoplasty in an Oriental population. Ann Plast Surg. 2005; 54:1–5.
Article5. Graham BS, Thiringer JK, Barrett TL. Nasal tip ulceration from infection and extrusion of a nasal alloplastic implant. J Am Acad Dermatol. 2001; 44:2 Suppl. 362–364.
Article6. Pak MW, Chan ES, van Hasselt CA. Late complications of nasal augmentation using silicone implants. J Laryngol Otol. 1998; 112:1074–1077.
Article7. Erlich MA, Parhiscar A. Nasal dorsal augmentation with silicone implants. Facial Plast Surg. 2003; 19:325–330.
Article8. McCurdy JA Jr. The Asian nose: augmentation rhinoplasty with L-shaped silicone implants. Facial Plast Surg. 2002; 18:245–252.
Article9. Godin MS, Waldman SR, Johnson CM Jr. Nasal augmentation using Gore-Tex. A 10-year experience. Arch Facial Plast Surg. 1999; 1:118–121.10. Conrad K, Gillman G. A 6-year experience with the use of expanded polytetrafluoroethylene in rhinoplasty. Plast Reconstr Surg. 1998; 101:1675–1683.
Article11. Jin HR, Lee JY, Yeon JY, Rhee CS. A multicenter evaluation of the safety of Gore-Tex as an implant in Asian rhinoplasty. Am J Rhinol. 2006; 20:615–619.
Article12. Jang TY, Choi JY, Jung DH, Park HJ, Lim SC. Histologic study of Gore-Tex removed after rhinoplasty. Laryngoscope. 2009; 119:620–627.
Article13. Yang SJ, Lee JH, Tark MS. Problems of expanded polytetrafluoroethylene (Gore-Tex(R)) in augmentation rhinoplasty. J Korean Soc Plast Reconstr Surg. 2004; 31:28–33.14. Hong JP, Yoon JY, Choi JW. Are polytetrafluoroethylene (Gore-Tex) implants an alternative material for nasal dorsal augmentation in Asians? J Craniofac Surg. 2010; 21:1750–1754.
Article15. Romo T 3rd, Kwak ES. Nasal grafts and implants in revision rhinoplasty. Facial Plast Surg Clin North Am. 2006; 14:373–387.
Article16. Mendelsohn M. Straightening the crooked middle third of the nose: using porous polyethylene extended spreader grafts. Arch Facial Plast Surg. 2005; 7:74–80.17. Gürlek A, Celik M, Fariz A, Ersöz-Oztürk A, Eren AT, Tenekeci G. The use of high-density porous polyethylene as a custom-made nasal spreader graft. Aesthetic Plast Surg. 2006; 30:34–41.
Article18. Romo T 3rd, Sclafani AP, Sabini P. Use of porous high-density polyethylene in revision rhinoplasty and in the platyrrhine nose. Aesthetic Plast Surg. 1998; 22:211–221.
Article19. Kim JG, Rhee SC, Cho PD, Kim DJ, Lee SH. Absorbable plate as a perpendicular strut for acute saddle nose deformities. Arch Plast Surg. 2012; 39:113–117.
Article20. Dhong ES, Kim YJ, Suh MK. L-shaped columellar strut in East asian nasal tip plasty. Arch Plast Surg. 2013; 40:616–620.
Article21. Winkler AA, Soler ZM, Leong PL, Murphy A, Wang TD, Cook TA. Complications associated with alloplastic implants in rhinoplasty. Arch Facial Plast Surg. 2012; 14:437–441.
Article22. Dingman RO, Grabb WC. Costal cartilage homografts preserved by irradiation. Plast Reconstr Surg Transplant Bull. 1961; 28:562–567.
Article23. Dingman RO, Grabb WC. [Follow-up clinic] Costal cartilage homografts presered by radiation. Plast Reconstr Surg. 1972; 50:516–517.24. Wainwright DJ. Use of an acellular allograft dermal matrix (AlloDerm) in the management of full-thickness burns. Burns. 1995; 21:243–248.
Article25. Davila AA, Seth AK, Wang E, Hanwright P, Bilimoria K, Fine N, et al. Human acellular dermis versus submuscular tissue expander breast reconstruction: a multivariate analysis of short-term complications. Arch Plast Surg. 2013; 40:19–27.
Article26. Lee JH, Park KR, Kim TG, Ha JH, Chung KJ, Kim YH, et al. A comparative study of CG CryoDerm and AlloDerm in direct-to-implant immediate breast reconstruction. Arch Plast Surg. 2013; 40:374–379.
Article27. Glaich AS, Cohen JL, Goldberg LH. Injection necrosis of the glabella: protocol for prevention and treatment after use of dermal fillers. Dermatol Surg. 2006; 32:276–281.
Article28. Lazzeri D, Agostini T, Figus M, Nardi M, Pantaloni M, Lazzeri S. Blindness following cosmetic injections of the face. Plast Reconstr Surg. 2012; 129:995–1012.
Article29. Oh S. Multiple embolism after injection of hyaluronic acid filler in nasal dorsum: a case report of skin necrosis, blindness, oculomotor palsy and cerebral infraction. Arch Aesthetic Plast Surg. 2012; 18:138–141.30. Peter S, Mennel S. Retinal branch artery occlusion following injection of hyaluronic acid (Restylane). Clin Experiment Ophthalmol. 2006; 34:363–364.
Article31. Sung MS, Kim HG, Woo KI, Kim YD. Ocular ischemia and ischemic oculomotor nerve palsy after vascular embolization of injectable calcium hydroxylapatite filler. Ophthal Plast Reconstr Surg. 2010; 26:289–291.
Article32. Christensen L. Normal and pathologic tissue reactions to soft tissue gel fillers. Dermatol Surg. 2007; 33:Suppl 2. S168–S175.
Article33. Lemperle G, Gauthier-Hazan N, Wolters M, Eisemann-Klein M, Zimmermann U, Duffy DM. Foreign body granulomas after all injectable dermal fillers: part 1. Possible causes. Plast Reconstr Surg. 2009; 123:1842–1863.
Article34. Sung HM, Suh IS, Lee HB, Tak KS, Moon KM, Jung MS. Case reports of adipose-derived stem cell therapy for nasal skin necrosis after filler injection. Arch Plast Surg. 2012; 39:51–54.
Article35. Choi J, Minn KW, Chang H. The efficacy and safety of platelet-rich plasma and adipose-derived stem cells: an update. Arch Plast Surg. 2012; 39:585–592.
Article36. Yang JD, Choi DS, Cho YK, Kim TK, Lee JW, Choi KY, et al. Effect of amniotic fluid stem cells and amniotic fluid cells on the wound healing process in a white rat model. Arch Plast Surg. 2013; 40:496–504.
Article37. Lee JW, Kwon OH, Kim TK, Cho YK, Choi KY, Chung HY, et al. Platelet-rich plasma: quantitative assessment of growth factor levels and comparative analysis of activated and inactivated groups. Arch Plast Surg. 2013; 40:530–535.
Article38. Salibian AA, Widgerow AD, Abrouk M, Evans GR. Stem cells in plastic surgery: a review of current clinical and translational applications. Arch Plast Surg. 2013; 40:666–675.
Article39. North JF. The use of preserved bovine cartilage in plastic surgery. Plast Reconstr Surg (1946). 1953; 11:261–274.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Infection of Tutoplast-Processed Fascia Lata in Augmentation Rhinoplasty
- Problems of Expanded Polytetrafluoroethylene (Gore-Tex(R)) in Augmentation Rhinoplasty
- Dorsal Augmentation in Secondary Rhinoplasty
- Rib/costal Cartilage Combination Graft in Rhinoplasty
- A Report on the Questionnaire about Augmentation Rhinoplasty