Yonsei Med J.
2014 Nov;55(6):1533-1541. 10.3349/ymj.2014.55.6.1533.
Cost-Effectiveness of Drug-Eluting vs. Bare-Metal Stents in Patients with Coronary Artery Disease from the Korean National Health Insurance Database
- Affiliations
-
- 1Department of Preventive Medicine and Public Health, Ajou University School of Medicine, Suwon, Korea. ajoujkh@ajou.ac.kr
- 2Division of Social Welfare, Baekseok University, Cheonan, Korea.
- 3Graduate School of Public Health, Ajou University, Suwon, Korea.
- KMID: 2070200
- DOI: http://doi.org/10.3349/ymj.2014.55.6.1533
Abstract
- PURPOSE
The aim of this study was to evaluate the cost-effectiveness of the use of drug-eluting stents (DESs), as compared with bare-metal stents (BMSs) in Korea.
MATERIALS AND METHODS
A retrospective cohort study was conducted between January 2000 and December 2007. Subjects were stent-treated for the first time between 2004 and 2005, with four years of follow-up (2004-2007) (n=43674). The incremental cost-effectiveness ratio (ICER) was used to calculate the costs of DESs compared with BMSs among patients with coronary artery disease (CAD). Cost-effectiveness was assessed with effectiveness defined as a reduction in major adverse cardiac events after six months and after one, two, three, and four years.
RESULTS
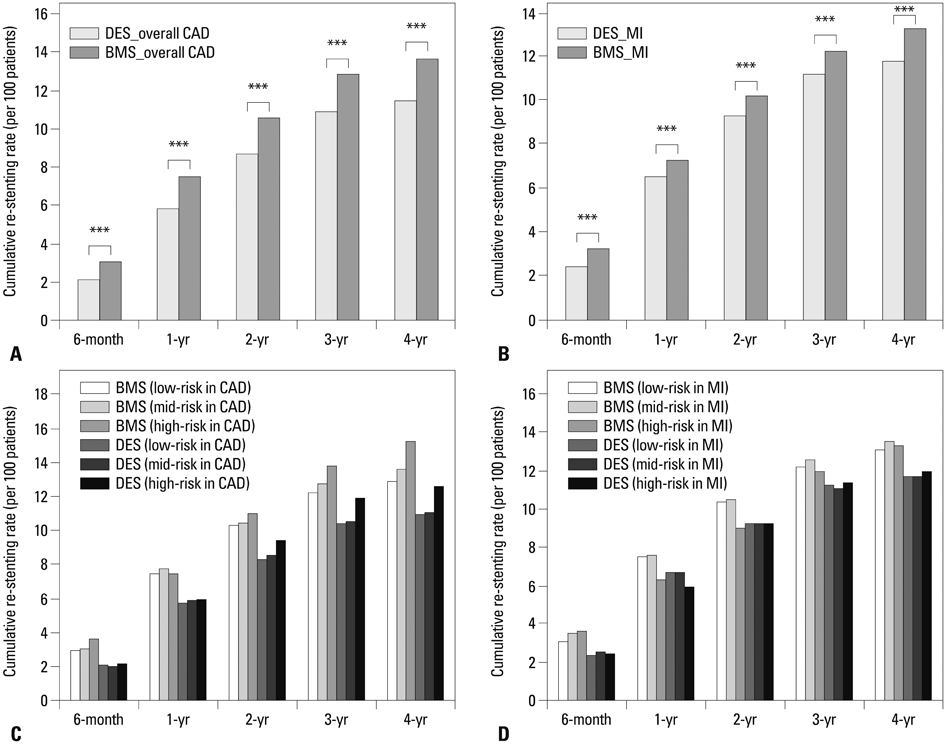
The total costs of a DESs were 674108 Korean won (KRW) higher than that of a BMSs at the end of the follow-up; 13635 thousand KRW per patient treated with DESs and 12960 thousand KRW per patient treated with BMSs. The ICER was 256315 per KRW/death avoided and 293090 per KRW/re-stenting avoided among the CAD patients at the end of the follow-up.
CONCLUSION
The ICER for the high-risk patients was lower than that for the low-risk patients. The use of DESs is clinically more useful than the use of BMSs for CAD and myocardial infarction patients, especially for those considered to be high-risk patients in Korea.
Keyword
MeSH Terms
-
Aged
*Angioplasty, Balloon, Coronary
Asian Continental Ancestry Group/statistics & numerical data
Coronary Artery Disease/etiology/*therapy
Cost-Benefit Analysis
Drug-Eluting Stents/economics
Female
Humans
Immunosuppressive Agents/administration & dosage/*economics
Male
Middle Aged
Myocardial Infarction/therapy
National Health Programs/*statistics & numerical data
Paclitaxel/administration & dosage
Republic of Korea/epidemiology
Retrospective Studies
Risk
Sirolimus/administration & dosage
Stents/adverse effects/*economics
Treatment Outcome
Immunosuppressive Agents
Paclitaxel
Sirolimus
Figure
Cited by 2 articles
-
Changes in the Practice of Coronary Revascularization between 2006 and 2010 in the Republic of Korea
Yoon Jung Choi, Jin-Bae Kim, Su-Jin Cho, Jaelim Cho, Jungwoo Sohn, Seong-Kyung Cho, Kyoung Hwa Ha, Changsoo Kim
Yonsei Med J. 2015;56(4):895-903. doi: 10.3349/ymj.2015.56.4.895.Therapeutic Hypothermia for Cardioprotection in Acute Myocardial Infarction
In Sook Kang, Ikeno Fumiaki, Wook Bum Pyun
Yonsei Med J. 2016;57(2):291-297. doi: 10.3349/ymj.2016.57.2.291.
Reference
-
1. Cho JS, Jeong MH, Jeong SY, Choi MJ, Chung JW, Hwang SH, et al. Predictive factor of the third coronary stent restenosis. Korean J Med. 2005; 69:255–263.2. Babapulle MN, Joseph L, Bélisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials of drug-eluting stents. Lancet. 2004; 364:583–591.
Article3. Jeong MH, Kim SH, Ahn YK, Cho JG, Park JC, Na KJ, et al. Predictive factors for the second restenosis after coronary interventions. Catheter Cardiovasc Interv. 2000; 50:34–39.
Article4. Kaiser C, Brunner-La Rocca HP, Buser PT, Bonetti PO, Osswald S, Linka A, et al. Incremental cost-effectiveness of drug-eluting stents compared with a third-generation bare-metal stent in a real-world setting: randomised Basel Stent Kosten Effektivitäts Trial (BASKET). Lancet. 2005; 366:921–929.
Article5. Brunner-La Rocca HP, Kaiser C, Bernheim A, Zellweger MJ, Jeger R, Buser PT, et al. Cost-effectiveness of drug-eluting stents in patients at high or low risk of major cardiac events in the Basel Stent KostenEffektivitäts Trial (BASKET): an 18-month analysis. Lancet. 2007; 370:1552–1559.
Article6. Stone GW, Grines CL, Cox DA, Garcia E, Tcheng JE, Griffin JJ, et al. Comparison of angioplasty with stenting, with or without abciximab, in acute myocardial infarction. N Engl J Med. 2002; 346:957–966.
Article7. Kwon S. Payment system reform for health care providers in Korea. Health Policy Plan. 2003; 18:84–92.
Article8. Lee K, Lee S. Effects of the DRG-based prospective payment system operated by the voluntarily participating providers on the cesarean section rates in Korea. Health Policy. 2007; 81:300–308.
Article9. Sundararajan V, Henderson T, Perry C, Muggivan A, Quan H, Ghali WA. New ICD-10 version of the Charlson comorbidity index predicted in-hospital mortality. J Clin Epidemiol. 2004; 57:1288–1294.
Article10. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987; 40:373–383.
Article11. Serruys PW, de Jaegere P, Kiemeneij F, Macaya C, Rutsch W, Heyndrickx G, et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent Study Group. N Engl J Med. 1994; 331:489–495.
Article12. Fischman DL, Leon MB, Baim DS, Schatz RA, Savage MP, Penn I, et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994; 331:496–501.
Article13. Hoffmann R, Mintz GS. Coronary in-stent restenosis-predictors, treatment and prevention. Eur Heart J. 2000; 21:1739–1749.
Article14. Morice MC, Colombo A, Meier B, Serruys P, Tamburino C, Guagliumi G, et al. Sirolimus- vs paclitaxel-eluting stents in de novo coronary artery lesions: the REALITY trial: a randomized controlled trial. JAMA. 2006; 295:895–904.
Article15. Cohen DJ, Bakhai A, Shi C, Githiora L, Lavelle T, Berezin RH, et al. Cost-effectiveness of sirolimus-eluting stents for treatment of complex coronary stenoses: results from the Sirolimus-Eluting Balloon Expandable Stent in the Treatment of Patients With De Novo Native Coronary Artery Lesions (SIRIUS) trial. Circulation. 2004; 110:508–514.
Article16. Costa JR Jr, Abizaid A, Feres F, Costa R, Seixas AC, Maia F, et al. EXCELLA First-in-Man (FIM) study: safety and efficacy of novolimus-eluting stent in de novo coronary lesions. EuroIntervention. 2008; 4:53–58.
Article17. van Hout BA, Serruys PW, Lemos PA, van den Brand MJ, van Es GA, Lindeboom WK, et al. One year cost effectiveness of sirolimus eluting stents compared with bare metal stents in the treatment of single native de novo coronary lesions: an analysis from the RAVEL trial. Heart. 2005; 91:507–512.
Article18. Bakhai A, Stone GW, Mahoney E, Lavelle TA, Shi C, Berezin RH, et al. Cost effectiveness of paclitaxel-eluting stents for patients undergoing percutaneous coronary revascularization: results from the TAXUS-IV Trial. J Am Coll Cardiol. 2006; 48:253–261.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Is There a Benefit of Drug-Eluting Stents Rather than Bare Metal Stents in Large Coronary Artery Lesions?
- Usefulness of Drug-Eluting Stents in Angioplasty and Stenting of the Vertebral Artery Origin : Comparison with Bare Stents: Clinical Research
- A Case of Neointimal Calcification in a Drug-Eluting Stent
- Current status of drug-eluting stents
- Risk of Stent Stenosis after Implanting a First-Generation Drug-Eluting Stent and Drug Balloon Angioplasty