Transarterial Chemoembolization Using Gelatin Sponges or Microspheres Plus Lipiodol-Doxorubicin versus Doxorubicin-Loaded Beads for the Treatment of Hepatocellular Carcinoma
- Affiliations
-
- 1Department of Diagnostic Radiology, National Cheng-Kung University Hospital, Tainan 704, Taiwan, R.O.C. taicheng100704@yahoo.com.tw
- 2Liver Cancer Collaborative Oncology Group, National Cheng-Kung University Hospital, Tainan 704, Taiwan, R.O.C.
- 3Department of Internal Medicine, National Cheng-Kung University Hospital, Tainan 704, Taiwan, R.O.C.
- KMID: 2069990
- DOI: http://doi.org/10.3348/kjr.2015.16.1.125
Abstract
OBJECTIVE
To retrospectively compare treatment of hepatocellular carcinoma (HCC) with transarterial chemoembolization (TACE) using gelatin sponges or microspheres plus lipiodol-doxorubicin vs. doxorubicin-loaded drug-eluting beads (DEB).
MATERIALS AND METHODS
A total of 158 patients with HCC received TACE from November 2010 to November 2011 were enrolled in this study, including 64 (40.5%) received TACE with lipiodol-doxorubicin and gelatin sponges (group A), 41 (25.9%) received TACE with lipiodol-doxorubicin and microspheres (group B), and 53 (33.5%) received TACE with doxorubicin-loaded DEB (group C). Tumor response and adverse events (AEs) were evaluated.
RESULTS
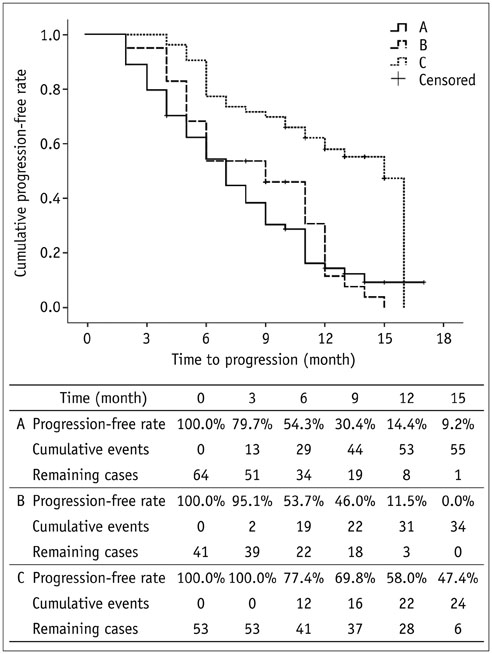
No significant difference was found at baseline among the three groups. The doxorubicin dosage in group C was significantly (p < 0.001) higher compared to the dose used in groups A or B (median, 50 mg vs. 31 mg or 25 mg). Significantly (p < 0.001) more patients in group C achieved complete response compared to those in groups A or B (32.1% vs. 6.3% or 2.4%). Significantly (p < 0.001) less patients in group C had progressive disease compared to those in groups A or B (34.0% vs. 57.8% or 68.3%). Minor AEs were more common in groups A and B compared to group C, with rates of 54.7%, 34.1%, and 5.7%, respectively.
CONCLUSION
In patients with HCC, TACE with DEB offers better safety and efficacy profiles compared to either TACE with gelatin sponges or TACE with microspheres.
MeSH Terms
-
Abdominal Pain/etiology
Adult
Aged
Antibiotics, Antineoplastic/*administration & dosage/adverse effects
Carcinoma, Hepatocellular/*drug therapy/mortality
Chemoembolization, Therapeutic
Disease-Free Survival
Doxorubicin/*administration & dosage/adverse effects
Drug Carriers/*chemistry
Ethiodized Oil/chemistry
Female
Fever/etiology
Follow-Up Studies
Gelatin/chemistry
Humans
Kaplan-Meier Estimate
Liver Neoplasms/*drug therapy/mortality
Male
Microspheres
Middle Aged
Retrospective Studies
Antibiotics, Antineoplastic
Drug Carriers
Ethiodized Oil
Doxorubicin
Gelatin
Figure
Cited by 3 articles
-
Optimized Performance of FlightPlan during Chemoembolization for Hepatocellular Carcinoma: Importance of the Proportion of Segmented Tumor Area
Seung-Moon Joo, Yong Pyo Kim, Tae Jun Yum, Na Lae Eun, Dahye Lee, Kwang-Hun Lee
Korean J Radiol. 2016;17(5):771-778. doi: 10.3348/kjr.2016.17.5.771.Comparison of the Efficacy and Prognostic Factors of Transarterial Chemoembolization Plus Microwave Ablation versus Transarterial Chemoembolization Alone in Patients with a Large Solitary or Multinodular Hepatocellular Carcinomas
Lin Zheng, Hai-Liang Li, Chen-Yang Guo, Su-Xia Luo
Korean J Radiol. 2018;19(2):237-246. doi: 10.3348/kjr.2018.19.2.237.Update on Transarterial Chemoembolization with Drug-Eluting Microspheres for Hepatocellular Carcinoma
Yasir M. Nouri, Jin Hyoung Kim, Hyun-Ki Yoon, Heung-Kyu Ko, Ji Hoon Shin, Dong Il Gwon
Korean J Radiol. 2019;20(1):34-49. doi: 10.3348/kjr.2018.0088.
Reference
-
1. Lewandowski RJ, Mulcahy MF, Kulik LM, Riaz A, Ryu RK, Baker TB, et al. Chemoembolization for hepatocellular carcinoma: comprehensive imaging and survival analysis in a 172-patient cohort. Radiology. 2010; 255:955–965.2. Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011; 61:69–90.3. Fan ST, Mau Lo C, Poon RT, Yeung C, Leung Liu C, Yuen WK, et al. Continuous improvement of survival outcomes of resection of hepatocellular carcinoma: a 20-year experience. Ann Surg. 2011; 253:745–758.4. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.5. Wu KT, Wang CC, Lu LG, Zhang WD, Zhang FJ, Shi F, et al. Hepatocellular carcinoma: clinical study of long-term survival and choice of treatment modalities. World J Gastroenterol. 2013; 19:3649–3657.6. Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003; 362:1907–1917.7. Marelli L, Stigliano R, Triantos C, Senzolo M, Cholongitas E, Davies N, et al. Transarterial therapy for hepatocellular carcinoma: which technique is more effective? A systematic review of cohort and randomized studies. Cardiovasc Intervent Radiol. 2007; 30:6–25.8. Thomas MB, Jaffe D, Choti MM, Belghiti J, Curley S, Fong Y, et al. Hepatocellular carcinoma: consensus recommendations of the National Cancer Institute Clinical Trials Planning Meeting. J Clin Oncol. 2010; 28:3994–4005.9. Ramsey DE, Kernagis LY, Soulen MC, Geschwind JF. Chemoembolization of hepatocellular carcinoma. J Vasc Interv Radiol. 2002; 13(9 Pt 2):S211–S221.10. Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003; 37:429–442.11. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008; 100:698–711.12. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010; 52:762–773.13. Brown DB, Gould JE, Gervais DA, Goldberg SN, Murthy R, Millward SF, et al. Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol. 2009; 20:7 Suppl. S425–S434.14. Lencioni R, de Baere T, Burrel M, Caridi JG, Lammer J, Malagari K, et al. Transcatheter treatment of hepatocellular carcinoma with Doxorubicin-loaded DC Bead (DEBDOX): technical recommendations. Cardiovasc Intervent Radiol. 2012; 35:980–985.15. Song MJ, Park CH, Kim JD, Kim HY, Bae SH, Choi JY, et al. Drug-eluting bead loaded with doxorubicin versus conventional Lipiodol-based transarterial chemoembolization in the treatment of hepatocellular carcinoma: a case-control study of Asian patients. Eur J Gastroenterol Hepatol. 2011; 23:521–527.16. Poyanli A, Rozaneş I, Acunaş B, Sencer S. Palliative treatment of hepatocellular carcinoma by chemoembolization. Acta Radiol. 2001; 42:602–607.17. Lee JK, Chung YH, Song BC, Shin JW, Choi WB, Yang SH, et al. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol. 2002; 17:52–58.18. Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007; 5:1100–1108.19. Nicolini A, Martinetti L, Crespi S, Maggioni M, Sangiovanni A. Transarterial chemoembolization with epirubicin-eluting beads versus transarterial embolization before liver transplantation for hepatocellular carcinoma. J Vasc Interv Radiol. 2010; 21:327–332.20. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007; 46:474–481.21. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010; 33:41–52.22. Granberg D, Eriksson LG, Welin S, Kindmark H, Janson ET, Skogseid B, et al. Liver embolization with trisacryl gelatin microspheres (embosphere) in patients with neuroendocrine tumors. Acta Radiol. 2007; 48:180–185.23. Covey AM, Brody LA, Maluccio MA, Getrajdman GI, Brown KT. Variant hepatic arterial anatomy revisited: digital subtraction angiography performed in 600 patients. Radiology. 2002; 224:542–547.24. de Baere T, Zhang X, Aubert B, Harry G, Lagrange C, Ropers J, et al. Quantification of tumor uptake of iodized oils and emulsions of iodized oils: experimental study. Radiology. 1996; 201:731–735.25. Idée JM, Guiu B. Use of Lipiodol as a drug-delivery system for transcatheter arterial chemoembolization of hepatocellular carcinoma: a review. Crit Rev Oncol Hematol. 2013; 88:530–549.26. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.27. Graf D, Vallböhmer D, Knoefel WT, Kröpil P, Antoch G, Sagir A, et al. Multimodal treatment of hepatocellular carcinoma. Eur J Intern Med. 2014; 25:430–437.28. Cardella JF, Kundu S, Miller DL, Millward SF, Sacks D. Society of Interventional Radiology. Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol. 2009; 20:7 Suppl. S189–S191.29. Nakamura H, Hashimoto T, Oi H, Sawada S. Transcatheter oily chemoembolization of hepatocellular carcinoma. Radiology. 1989; 170(3 Pt 1):783–786.30. Malagari K, Alexopoulou E, Chatzimichail K, Hall B, Koskinas J, Ryan S, et al. Transcatheter chemoembolization in the treatment of HCC in patients not eligible for curative treatments: midterm results of doxorubicin-loaded DC bead. Abdom Imaging. 2008; 33:512–519.31. Song DS, Choi JY, Yoo SH, Kim HY, Song MJ, Bae SH, et al. DC bead transarterial chemoembolization is effective in hepatocellular carcinoma refractory to conventional transarteral chemoembolization: a pilot study. Gut Liver. 2013; 7:89–95.32. Kalva SP, Pectasides M, Liu R, Rachamreddy N, Surakanti S, Yeddula K, et al. Safety and effectiveness of chemoembolization with drug-eluting beads for advanced-stage hepatocellular carcinoma. Cardiovasc Intervent Radiol. 2014; 37:381–387.33. Sacco R, Bargellini I, Bertini M, Bozzi E, Romano A, Petruzzi P, et al. Conventional versus doxorubicin-eluting bead transarterial chemoembolization for hepatocellular carcinoma. J Vasc Interv Radiol. 2011; 22:1545–1552.34. Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC). J Surg Oncol. 2010; 101:476–480.35. Malagari K, Pomoni M, Spyridopoulos TN, Moschouris H, Kelekis A, Dourakis S, et al. Safety profile of sequential transcatheter chemoembolization with DC Bead™: results of 237 hepatocellular carcinoma (HCC) patients. Cardiovasc Intervent Radiol. 2011; 34:774–785.36. Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006; 12:2563–2567.37. Wigmore SJ, Redhead DN, Thomson BN, Currie EJ, Parks RW, Madhavan KK, et al. Postchemoembolisation syndrome--tumour necrosis or hepatocyte injury? Br J Cancer. 2003; 89:1423–1427.38. Recchia F, Passalacqua G, Filauri P, Doddi M, Boscarato P, Candeloro G, et al. Chemoembolization of unresectable hepatocellular carcinoma: decreased toxicity with slow-release doxorubicineluting beads compared with lipiodol. Oncol Rep. 2012; 27:1377–1383.39. Vogl TJ, Lammer J, Lencioni R, Malagari K, Watkinson A, Pilleul F, et al. Liver, gastrointestinal, and cardiac toxicity in intermediate hepatocellular carcinoma treated with PRECISION TACE with drug-eluting beads: results from the PRECISION V randomized trial. AJR Am J Roentgenol. 2011; 197:W562–W570.40. López-Benítez R, Radeleff BA, Barragán-Campos HM, Noeldge G, Grenacher L, Richter GM, et al. Acute pancreatitis after embolization of liver tumors: frequency and associated risk factors. Pancreatology. 2007; 7:53–62.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: Now and future
- An Experimental Study on Mixing of Chemoembolic Material for Hepatocellular Carcinoma
- Therapeutic Effect of Transarterial Chemoembolization in Hepatocellular Carcinoma
- A Case of Cryptogenic Organizing Pneumonia after Transarterial Chemoembolization for the Treatment of Hepatocellular Carcinoma
- An A to Z of Lipiodol Beyond the Clinical Practice in the Management of Hepatocellular Carcinoma