Yonsei Med J.
2014 May;55(3):709-714. 10.3349/ymj.2014.55.3.709.
Thalamocortical Connections between the Mediodorsal Nucleus of the Thalamus and Prefrontal Cortex in the Human Brain: A Diffusion Tensor Tractographic Study
- Affiliations
-
- 1Department of Physical Medicine and Rehabilitation, College of Medicine, Yeungnam University, Daegu, Korea. eangbul@hanmail.net
- KMID: 2068681
- DOI: http://doi.org/10.3349/ymj.2014.55.3.709
Abstract
- PURPOSE
The elucidation of thalamocortical connections between the mediodorsal nucleus (MD) of thalamus and the prefrontal cortex (PFC) is important in the clinical fields of neurorehabilitation and psychiatry. However, little is known about these connections in human brain. We attempted to identify and investigate the anatomical characteristics of the thalamocortical connection between MD and PFC in human brain using diffusion tensor tractography (DTT).
MATERIALS AND METHODS
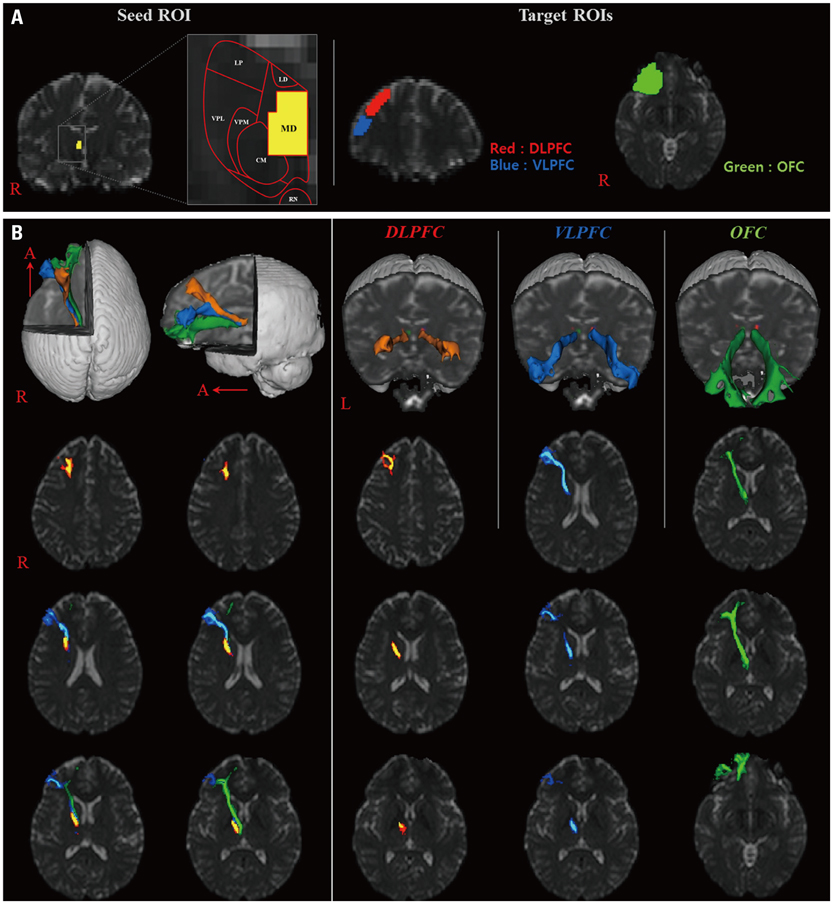
Thirty-two healthy volunteers were recruited for this study. Diffusion tensor images were scanned using a 1.5-T. A seed region of interest was placed at the MD of the thalamus on coronal images, and target regions of interest were placed on the dorsolateral prefrontal cortex (DLPFC), the ventrolateral prefrontal cortex (VLPFC), and the orbitofrontal cortex (OFC), respectively. The three thalamocortical connections found were reconstructed using Functional Magnetic Resonance Imaging of the Brain (FMRIB) software.
RESULTS
The three thalamocortical connections were arranged in subcortical white matter in the following order from upper to lower levels: the DLPFC, the VLPFC, and the OFC. In terms of fractional anisotropy and mean diffusivity values, no significant differences were observed between the DLPFC, VLPFC and OFC (p>0.05). In contrast, the OFC tract volume was higher than those of the DLPFC and the VLPFC (p<0.05).
CONCLUSION
Three thalamocortical connections were reconstructed between MD and PFCs in human brain using DTT. We believe that the results of this study would be helpful to clinicians in treating frontal network syndrome and psychiatric diseases.
MeSH Terms
Figure
Reference
-
1. Mesulam MM. Principles of behavioral and cognitive neurology. 2nd ed. New York: Oxford University;2000.2. Clark DL, Boutros NN, Mendez MF. The brain and behavior: an introduction to behavioral neuroanatomy. 3rd ed. New York: Cambridge University;2010.3. Sakagami M, Pan X. Functional role of the ventrolateral prefrontal cortex in decision making. Curr Opin Neurobiol. 2007; 17:228–233.
Article4. Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence. Science. 2000; 289:591–594.
Article5. Funahashi S. Prefrontal cortex and working memory processes. Neuroscience. 2006; 139:251–261.
Article6. Fuster JM. The Prefrontal Cortex. 4th ed. New York: Academic Press;2008.7. De Witte L, Brouns R, Kavadias D, Engelborghs S, De Deyn PP, Mariën P. Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex. 2011; 47:273–319.
Article8. Liebermann D, Ploner CJ, Kraft A, Kopp UA, Ostendorf F. A dysexecutive syndrome of the medial thalamus. Cortex. 2013; 49:40–49.
Article9. Summers MJ. Neuropsychological consequences of right thalamic haemorrhage: case study and review. Brain Cogn. 2002; 50:129–138.
Article10. Rose SE, Chalk JB, Janke AL, Strudwick MW, Windus LC, Hannah DE, et al. Evidence of altered prefrontal-thalamic circuitry in schizophrenia: an optimized diffusion MRI study. Neuroimage. 2006; 32:16–22.
Article11. Ray JP, Price JL. The organization of projections from the mediodorsal nucleus of the thalamus to orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol. 1993; 337:1–31.
Article12. Barbas H, Henion TH, Dermon CR. Diverse thalamic projections to the prefrontal cortex in the rhesus monkey. J Comp Neurol. 1991; 313:65–94.
Article13. Goldman-Rakic PS, Porrino LJ. The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol. 1985; 242:535–560.
Article14. Giguere M, Goldman-Rakic PS. Mediodorsal nucleus: areal, laminar, and tangential distribution of afferents and efferents in the frontal lobe of rhesus monkeys. J Comp Neurol. 1988; 277:195–213.
Article15. Reep RL, Corwin JV, King V. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp Brain Res. 1996; 111:215–232.
Article16. Ongür D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000; 10:206–219.
Article17. Contini M, Baccarini M, Borra E, Gerbella M, Rozzi S, Luppino G. Thalamic projections to the macaque caudal ventrolateral prefrontal areas 45A and 45B. Eur J Neurosci. 2010; 32:1337–1353.
Article18. Eckert U, Metzger CD, Buchmann JE, Kaufmann J, Osoba A, Li M, et al. Preferential networks of the mediodorsal nucleus and centromedian-parafascicular complex of the thalamus--a DTI tractography study. Hum Brain Mapp. 2012; 33:2627–2637.
Article19. Klein JC, Rushworth MF, Behrens TE, Mackay CE, de Crespigny AJ, D'Arceuil H, et al. Topography of connections between human prefrontal cortex and mediodorsal thalamus studied with diffusion tractography. Neuroimage. 2010; 51:555–564.
Article20. Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR, et al. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Res. 2010; 183:144–150.
Article21. Wakana S, Jiang H, Nagae-Poetscher LM, van Zijl PC, Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004; 230:77–87.
Article22. Nagae LM, Hoon AH Jr, Stashinko E, Lin D, Zhang W, Levey E, et al. Diffusion tensor imaging in children with periventricular leukomalacia: variability of injuries to white matter tracts. AJNR Am J Neuroradiol. 2007; 28:1213–1222.
Article23. Yin H, Cheng SH, Zhang J, Ma L, Gao Y, Li D, et al. Corticospinal tract degeneration in amyotrophic lateral sclerosis: a diffusion tensor imaging and fibre tractography study. Ann Acad Med Singapore. 2008; 37:411–415.24. Kwon HG, Hong JH, Hong CP, Lee DH, Ahn SH, Jang SH. Dentatorubrothalamic tract in human brain: diffusion tensor tractography study. Neuroradiology. 2011; 53:787–791.
Article25. Kwon HG, Hong JH, Jang SH. Mammillothalamic tract in human brain: diffusion tensor tractography study. Neurosci Lett. 2010; 481:51–53.
Article26. Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004; 23:Suppl 1. S208–S219.
Article27. Johansen-Berg H, Behrens TE, Sillery E, Ciccarelli O, Thompson AJ, Smith SM, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005; 15:31–39.
Article28. Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos Trans R Soc Lond B Biol Sci. 2005; 360:781–795.
Article29. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005; 6:691–702.
Article30. Kubota M, Miyata J, Sasamoto A, Sugihara G, Yoshida H, Kawada R, et al. Thalamocortical disconnection in the orbitofrontal region associated with cortical thinning in schizophrenia. JAMA Psychiatry. 2013; 70:12–21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Injury of the Thalamocortical Pathway Between the Mediodorsal Nuclei and the Prefrontal Cortex in a Patient with Traumatic Brain Injury
- The Upper Ascending Reticular Activating System between Intralaminar Thalamic Nuclei and Cerebral Cortex in the Human Brain
- Electroencephalographic Response of Thalamic Hypothalamic Modulation System on Coagulation or Stimulation of Thalamus and Hypothalamus in Dogs
- Association of Dysphagia With Supratentorial Lesions in Patients With Middle Cerebral Artery Stroke
- Diffusion Tensor Imaging: Exploring the Motor Networks and Clinical Applications