Yonsei Med J.
2014 May;55(3):689-699. 10.3349/ymj.2014.55.3.689.
Agmatine Improves Cognitive Dysfunction and Prevents Cell Death in a Streptozotocin-Induced Alzheimer Rat Model
- Affiliations
-
- 1Department of Anatomy, Yonsei University College of Medicine, Seoul, Korea. jelee@yuhs.ac
- 2Brain Korea 21 Plus Project for Medical Science, Brain Research Institute, Yonsei University College of Medicine, Seoul, Korea.
- 3Department of Neurology, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 2068679
- DOI: http://doi.org/10.3349/ymj.2014.55.3.689
Abstract
- PURPOSE
Alzheimer's disease (AD) results in memory impairment and neuronal cell death in the brain. Previous studies demonstrated that intracerebroventricular administration of streptozotocin (STZ) induces pathological and behavioral alterations similar to those observed in AD. Agmatine (Agm) has been shown to exert neuroprotective effects in central nervous system disorders. In this study, we investigated whether Agm treatment could attenuate apoptosis and improve cognitive decline in a STZ-induced Alzheimer rat model.
MATERIALS AND METHODS
We studied the effect of Agm on AD pathology using a STZ-induced Alzheimer rat model. For each experiment, rats were given anesthesia (chloral hydrate 300 mg/kg, ip), followed by a single injection of STZ (1.5 mg/kg) bilaterally into each lateral ventricle (5 microL/ventricle). Rats were injected with Agm (100 mg/kg) daily up to two weeks from the surgery day.
RESULTS
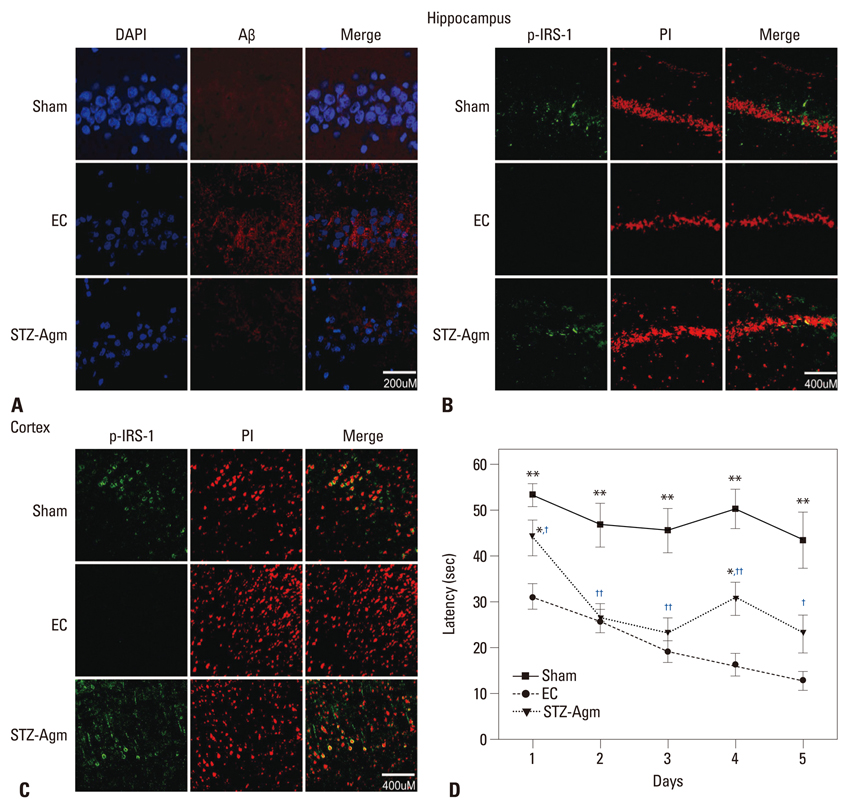
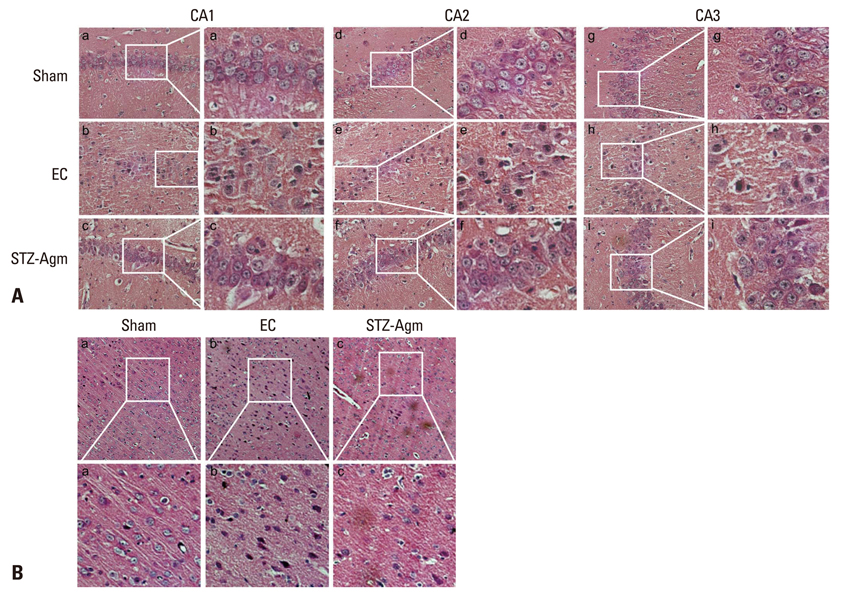
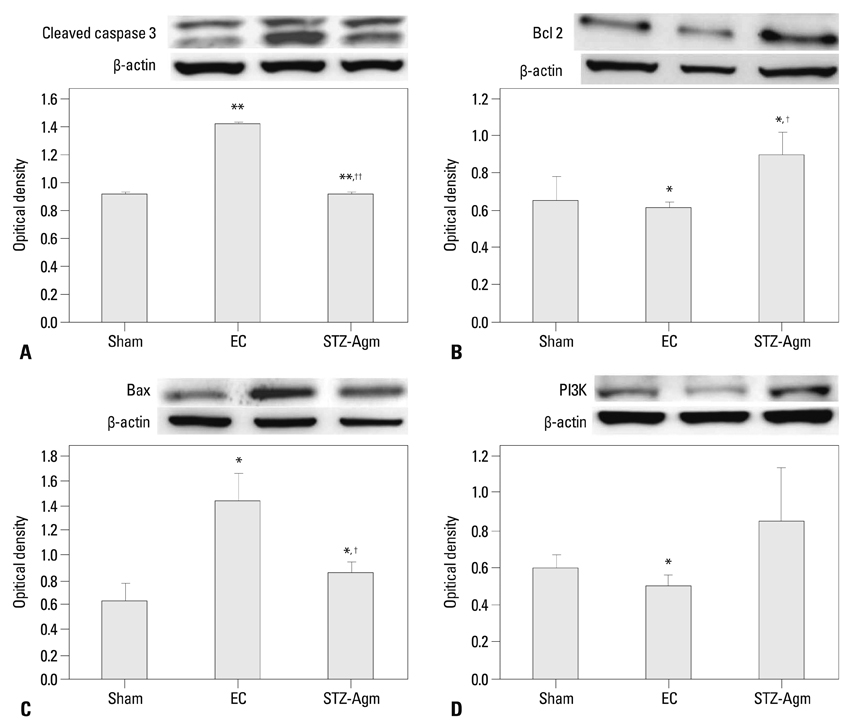
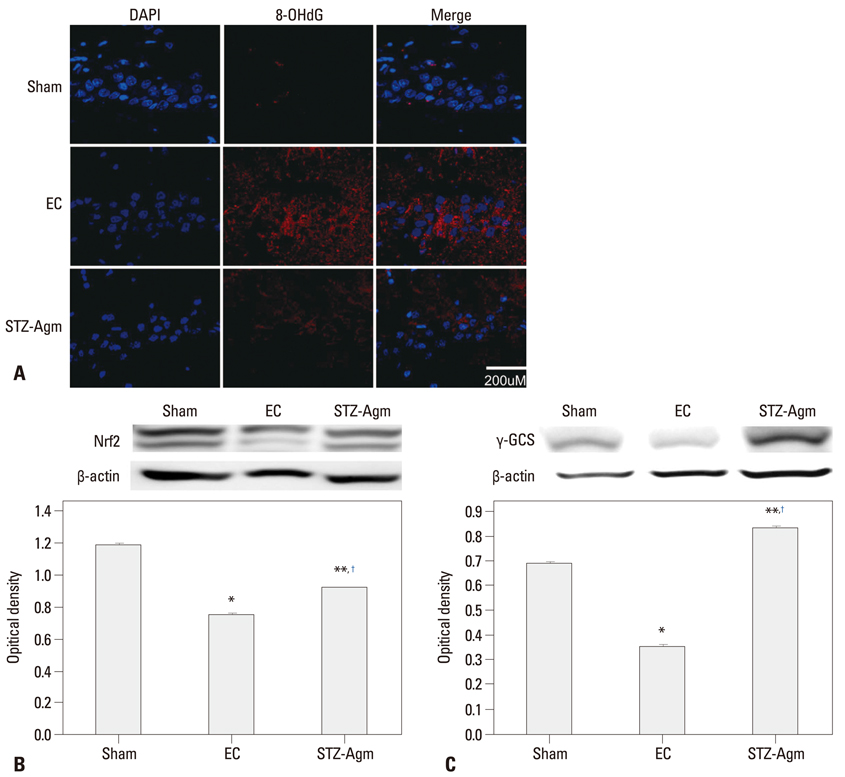
Agm suppressed the accumulation of amyloid beta and enhanced insulin signal transduction in STZ-induced Alzheimer rats [experimetal control (EC) group]. Upon evaluation of cognitive function by Morris water maze testing, significant improvement of learning and memory dysfunction in the STZ-Agm group was observed compared with the EC group. Western blot results revealed significant attenuation of the protein expressions of cleaved caspase-3 and Bax, as well as increases in the protein expressions of Bcl2, PI3K, Nrf2, and gamma-glutamyl cysteine synthetase, in the STZ-Agm group.
CONCLUSION
Our results showed that Agm is involved in the activation of antioxidant signaling pathways and activation of insulin signal transduction. Accordingly, Agm may be a promising therapeutic agent for improving cognitive decline and attenuating apoptosis in AD.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Metabolism-Centric Overview of the Pathogenesis of Alzheimer's Disease
Somang Kang, Yong-ho Lee, Jong Eun Lee
Yonsei Med J. 2017;58(3):479-488. doi: 10.3349/ymj.2017.58.3.479.Role of agmatine in the application of neural progenitor cell in central nervous system diseases: therapeutic potentials and effects
Renée Kosonen, Sumit Barua, Jong Youl Kim, Jong Eun Lee
Anat Cell Biol. 2021;54(2):143-151. doi: 10.5115/acb.21.089.
Reference
-
1. Hyman BT, Damasio H, Damasio AR, Van Hoesen GW. Alzheimer's disease. Annu Rev Public Health. 1989; 10:115–140.
Article2. Van Hoesen GW, Augustinack JC, Dierking J, Redman SJ, Thangavel R. The parahippocampal gyrus in Alzheimer's disease. Clinical and preclinical neuroanatomical correlates. Ann N Y Acad Sci. 2000; 911:254–274.
Article3. Arispe N, Rojas E, Pollard HB. Alzheimer disease amyloid beta protein forms calcium channels in bilayer membranes: blockade by tromethamine and aluminum. Proc Natl Acad Sci U S A. 1993; 90:567–571.
Article4. Furukawa K, Abe Y, Akaike N. Amyloid beta protein-induced irreversible current in rat cortical neurones. Neuroreport. 1994; 5:2016–2018.
Article5. Mattson MP, Barger SW, Cheng B, Lieberburg I, Smith-Swintosky VL, Rydel RE. beta-Amyloid precursor protein metabolites and loss of neuronal Ca2+ homeostasis in Alzheimer's disease. Trends Neurosci. 1993; 16:409–414.
Article6. Alberdi E, Sánchez-Gómez MV, Cavaliere F, Pérez-Samartín A, Zugaza JL, Trullas R, et al. Amyloid beta oligomers induce Ca2+ dysregulation and neuronal death through activation of ionotropic glutamate receptors. Cell Calcium. 2010; 47:264–272.
Article7. Kelly BL, Ferreira A. beta-Amyloid-induced dynamin 1 degradation is mediated by N-methyl-D-aspartate receptors in hippocampal neurons. J Biol Chem. 2006; 281:28079–28089.
Article8. Lester-Coll N, Rivera EJ, Soscia SJ, Doiron K, Wands JR, de la Monte SM. Intracerebral streptozotocin model of type 3 diabetes: relevance to sporadic Alzheimer's disease. J Alzheimers Dis. 2006; 9:13–33.
Article9. Butterfield DA. Proteomics: a new approach to investigate oxidative stress in Alzheimer's disease brain. Brain Res. 2004; 1000:1–7.
Article10. Lannert H, Hoyer S. Intracerebroventricular administration of streptozotocin causes long-term diminutions in learning and memory abilities and in cerebral energy metabolism in adult rats. Behav Neurosci. 1998; 112:1199–1208.
Article11. Gibson GE, Huang HM. Oxidative stress in Alzheimer's disease. Neurobiol Aging. 2005; 26:575–578.
Article12. Grünblatt E, Salkovic-Petrisic M, Osmanovic J, Riederer P, Hoyer S. Brain insulin system dysfunction in streptozotocin intracerebroventricularly treated rats generates hyperphosphorylated tau protein. J Neurochem. 2007; 101:757–770.
Article13. Weinstock M, Shoham S. Rat models of dementia based on reductions in regional glucose metabolism, cerebral blood flow and cytochrome oxidase activity. J Neural Transm. 2004; 111:347–366.
Article14. Dröge W, Kinscherf R. Aberrant insulin receptor signaling and amino acid homeostasis as a major cause of oxidative stress in aging. Antioxid Redox Signal. 2008; 10:661–678.
Article15. Hoyer S, Lannert H. Inhibition of the neuronal insulin receptor causes Alzheimer-like disturbances in oxidative/energy brain metabolism and in behavior in adult rats. Ann N Y Acad Sci. 1999; 893:301–303.
Article16. Hoyer S. Glucose metabolism and insulin receptor signal transduction in Alzheimer disease. Eur J Pharmacol. 2004; 490:115–125.
Article17. Ahmad M, Saleem S, Ahmad AS, Yousuf S, Ansari MA, Khan MB, et al. Ginkgo biloba affords dose-dependent protection against 6-hydroxydopamine-induced parkinsonism in rats: neurobehavioural, neurochemical and immunohistochemical evidences. J Neurochem. 2005; 93:94–104.
Article18. Ansari MA, Ahmad AS, Ahmad M, Salim S, Yousuf S, Ishrat T, et al. Selenium protects cerebral ischemia in rat brain mitochondria. Biol Trace Elem Res. 2004; 101:73–86.
Article19. Arteni NS, Lavinsky D, Rodrigues AL, Frison VB, Netto CA. Agmatine facilitates memory of an inhibitory avoidance task in adult rats. Neurobiol Learn Mem. 2002; 78:465–469.
Article20. Liu P, Collie ND. Behavioral effects of agmatine in naive rats are task- and delay-dependent. Neuroscience. 2009; 163:82–96.
Article21. Lu W, Dong HJ, Gong ZH, Su RB, Li J. Agmatine inhibits morphine-induced memory impairment in the mouse step-down inhibitory avoidance task. Pharmacol Biochem Behav. 2010; 97:256–261.
Article22. McKay BE, Lado WE, Martin LJ, Galic MA, Fournier NM. Learning and memory in agmatine-treated rats. Pharmacol Biochem Behav. 2002; 72:551–557.
Article23. Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N, et al. Agmatine prevents LPS-induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol. 2010; 634:84–88.
Article24. Feng Y, Piletz JE, Leblanc MH. Agmatine suppresses nitric oxide production and attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res. 2002; 52:606–611.
Article25. Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004; 189:122–130.
Article26. Regunathan S, Dozier D, Takkalapalli R, Phillips WJ. Agmatine levels in the cerebrospinal fluid of normal human volunteers. J Pain Palliat Care Pharmacother. 2009; 23:35–39.
Article27. Reis DJ, Regunathan S. Agmatine: a novel neurotransmitter? Adv Pharmacol. 1998; 42:645–649.
Article28. Regunathan S, Youngson C, Raasch W, Wang H, Reis DJ. Imidazoline receptors and agmatine in blood vessels: a novel system inhibiting vascular smooth muscle proliferation. J Pharmacol Exp Ther. 1996; 276:1272–1282.29. Halaris A, Plietz J. Agmatine: metabolic pathway and spectrum of activity in brain. CNS Drugs. 2007; 21:885–900.30. Reis DJ, Regunathan S. Is agmatine a novel neurotransmitter in brain? Trends Pharmacol Sci. 2000; 21:187–193.
Article31. Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL. Nrf2, a Cap'n'Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem. 1999; 274:26071–26078.
Article32. Motohashi H, Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med. 2004; 10:549–557.
Article33. Ramprasath T, Selvam GS. Potential impact of genetic variants in Nrf2 regulated antioxidant genes and risk prediction of diabetes and associated cardiac complications. Curr Med Chem. 2013; 20:4680–4693.
Article34. Wild AC, Gipp JJ, Mulcahy T. Overlapping antioxidant response element and PMA response element sequences mediate basal and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase catalytic subunit gene. Biochem J. 1998; 332(Pt 2):373–381.
Article35. Tomobe K, Shinozuka T, Kuroiwa M, Nomura Y. Age-related changes of Nrf2 and phosphorylated GSK-3β in a mouse model of accelerated aging (SAMP8). Arch Gerontol Geriatr. 2012; 54:e1–e7.
Article36. Venugopal R, Jaiswal AK. Nrf1 and Nrf2 positively and c-Fos and Fra1 negatively regulate the human antioxidant response element-mediated expression of NAD(P)H:quinone oxidoreductase1 gene. Proc Natl Acad Sci U S A. 1996; 93:14960–14965.
Article37. Kanninen K, White AR, Koistinaho J, Malm T. Targeting Glycogen Synthase Kinase-3β for Therapeutic Benefit against Oxidative Stress in Alzheimer's Disease: Involvement of the Nrf2-ARE Pathway. Int J Alzheimers Dis. 2011; 2011:985085.38. Li XH, Li CY, Xiang ZG, Hu JJ, Lu JM, Tian RB, et al. Allicin ameliorates cardiac hypertrophy and fibrosis through enhancing of Nrf2 antioxidant signaling pathways. Cardiovasc Drugs Ther. 2012; 26:457–465.
Article39. Yang Y, Zhang J, Liu H, Zhang L. Change of Nrf2 expression in rat hippocampus in a model of chronic cerebral hypoperfusion. Int J Neurosci. 2013; [Epub ahead of print].
Article40. Rodrigues L, Dutra MF, Ilha J, Biasibetti R, Quincozes-Santos A, Leite MC, et al. Treadmill training restores spatial cognitive deficits and neurochemical alterations in the hippocampus of rats submitted to an intracerebroventricular administration of streptozotocin. J Neural Transm. 2010; 117:1295–1305.
Article41. Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984; 11:47–60.
Article42. Paul CA, Beltz B, Berger-Sweeney J. Perfusion of brain tissues with fixative. CSH Protoc. 2008; 2008:pdb.prot4802.
Article43. Flynn BL. Pharmacologic management of Alzheimer disease, Part I: Hormonal and emerging investigational drug therapies. Ann Pharmacother. 1999; 33:178–187.
Article44. Sharma M, Gupta YK. Chronic treatment with trans resveratrol prevents intracerebroventricular streptozotocin induced cognitive impairment and oxidative stress in rats. Life Sci. 2002; 71:2489–2498.
Article45. Selkoe DJ. Alzheimer's disease results from the cerebral accumulation and cytotoxicity of amyloid beta-protein. J Alzheimers Dis. 2001; 3:75–80.
Article46. Salkovic-Petrisic M, Hoyer S. Central insulin resistance as a trigger for sporadic Alzheimer-like pathology: an experimental approach. J Neural Transm Suppl. 2007; 217–233.
Article47. Maftei M, Thurm F, Schnack C, Tumani H, Otto M, Elbert T, et al. Increased levels of antigen-bound β-amyloid autoantibodies in serum and cerebrospinal fluid of Alzheimer's disease patients. PLoS One. 2013; 8:e68996.
Article48. Selkoe DJ. Alzheimer's disease: a central role for amyloid. J Neuropathol Exp Neurol. 1994; 53:438–447.
Article49. Varadarajan S, Yatin S, Aksenova M, Butterfield DA. Review: Alzheimer's amyloid beta-peptide-associated free radical oxidative stress and neurotoxicity. J Struct Biol. 2000; 130:184–208.
Article50. Varadarajan S, Kanski J, Aksenova M, Lauderback C, Butterfield DA. Different mechanisms of oxidative stress and neurotoxicity for Alzheimer's A beta(1--42) and A beta(25--35). J Am Chem Soc. 2001; 123:5625–5631.
Article51. Kornhuber J, Bormann J, Retz W, Hübers M, Riederer P. Memantine displaces [3H]MK-801 at therapeutic concentrations in postmortem human frontal cortex. Eur J Pharmacol. 1989; 166:589–590.
Article52. Piletz JE, Aricioglu F, Cheng JT, Fairbanks CA, Gilad VH, Haenisch B, et al. Agmatine: clinical applications after 100 years in translation. Drug Discov Today. 2013; 18:880–893.
Article53. Salloway S, Mintzer J, Weiner MF, Cummings JL. Disease-modifying therapies in Alzheimer's disease. Alzheimers Dement. 2008; 4:65–79.
Article54. Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY. Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res. 2006; 1084:210–216.
Article55. O'Neill C, Kiely AP, Coakley MF, Manning S, Long-Smith CM. Insulin and IGF-1 signalling: longevity, protein homoeostasis and Alzheimer's disease. Biochem Soc Trans. 2012; 40:721–727.56. Freude S, Hettich MM, Schumann C, Stöhr O, Koch L, Köhler C, et al. Neuronal IGF-1 resistance reduces Abeta accumulation and protects against premature death in a model of Alzheimer's disease. FASEB J. 2009; 23:3315–3324.
Article57. Craft S, Asthana S, Newcomer JW, Wilkinson CW, Matos IT, Baker LD, et al. Enhancement of memory in Alzheimer disease with insulin and somatostatin, but not glucose. Arch Gen Psychiatry. 1999; 56:1135–1140.
Article58. Park CR, Seeley RJ, Craft S, Woods SC. Intracerebroventricular insulin enhances memory in a passive-avoidance task. Physiol Behav. 2000; 68:509–514.
Article59. van der Heide LP, Ramakers GM, Smidt MP. Insulin signaling in the central nervous system: learning to survive. Prog Neurobiol. 2006; 79:205–221.
Article60. Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994; 369:233–235.
Article61. van der Heide LP, Kamal A, Artola A, Gispen WH, Ramakers GM. Insulin modulates hippocampal activity-dependent synaptic plasticity in a N-methyl-d-aspartate receptor and phosphatidyl-inositol-3-kinase-dependent manner. J Neurochem. 2005; 94:1158–1166.
Article62. Zhao WQ, Chen H, Quon MJ, Alkon DL. Insulin and the insulin receptor in experimental models of learning and memory. Eur J Pharmacol. 2004; 490:71–81.
Article63. Park CR. Cognitive effects of insulin in the central nervous system. Neurosci Biobehav Rev. 2001; 25:311–323.
Article64. Liu Y, Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Deficient brain insulin signalling pathway in Alzheimer's disease and diabetes. J Pathol. 2011; 225:54–62.
Article65. Ahn SK, Hong S, Park YM, Choi JY, Lee WT, Park KA, et al. Protective effects of agmatine on lipopolysaccharide-injured microglia and inducible nitric oxide synthase activity. Life Sci. 2012; 91:1345–1350.
Article66. Bokara KK, Kwon KH, Nho Y, Lee WT, Park KA, Lee JE. Retroviral expression of arginine decarboxylase attenuates oxidative burden in mouse cortical neural stem cells. Stem Cells Dev. 2011; 20:527–537.
Article67. Seo SK, Yang W, Park YM, Lee WT, Park KA, Lee JE. Overexpression of human arginine decarboxylase rescues human mesenchymal stem cells against H2O2 toxicity through cell survival protein activation. J Korean Med Sci. 2013; 28:366–373.
Article68. Liu P, Bergin DH. Differential effects of i.c.v. microinfusion of agmatine on spatial working and reference memory in the rat. Neuroscience. 2009; 159:951–961.
Article69. Frölich L, Riederer P. Free radical mechanisms in dementia of Alzheimer type and the potential for antioxidative treatment. Arzneimittelforschung. 1995; 45:443–446.70. Launer LJ, Kalmijn S. Anti-oxidants and cognitive function: a review of clinical and epidemiologic studies. J Neural Transm Suppl. 1998; 53:1–8.
Article71. Mikati MA, Abi-Habib RJ, El Sabban ME, Dbaibo GS, Kurdi RM, Kobeissi M, et al. Hippocampal programmed cell death after status epilepticus: evidence for NMDA-receptor and ceramide-mediated mechanisms. Epilepsia. 2003; 44:282–291.
Article72. Brouillette J. The Effects of Soluble Aβ Oligomers on Neurodegeneration in Alzheimer's Disease. Curr Pharm Des. 2013; [Epub ahead of print].73. Texidó L, Martín-Satué M, Alberdi E, Solsona C, Matute C. Amyloid β peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011; 49:184–190.
Article74. Feng Z, Zhang JT. Protective effect of melatonin on beta-amyloid-induced apoptosis in rat astroglioma C6 cells and its mechanism. Free Radic Biol Med. 2004; 37:1790–1801.
Article75. Montiel T, Quiroz-Baez R, Massieu L, Arias C. Role of oxidative stress on beta-amyloid neurotoxicity elicited during impairment of energy metabolism in the hippocampus: protection by antioxidants. Exp Neurol. 2006; 200:496–508.
Article76. Pavlik VN, Doody RS, Rountree SD, Darby EJ. Vitamin E use is associated with improved survival in an Alzheimer's disease cohort. Dement Geriatr Cogn Disord. 2009; 28:536–540.
Article77. Sano M, Ernesto C, Thomas RG, Klauber MR, Schafer K, Grundman M, et al. A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer's disease. The Alzheimer's Disease Cooperative Study. N Engl J Med. 1997; 336:1216–1222.
Article78. Ishrat T, Khan MB, Hoda MN, Yousuf S, Ahmad M, Ansari MA, et al. Coenzyme Q10 modulates cognitive impairment against intracerebroventricular injection of streptozotocin in rats. Behav Brain Res. 2006; 171:9–16.
Article79. Pathan AR, Viswanad B, Sonkusare SK, Ramarao P. Chronic administration of pioglitazone attenuates intracerebroventricular streptozotocin induced-memory impairment in rats. Life Sci. 2006; 79:2209–2216.
Article80. Sharma M, Gupta YK. Intracerebroventricular injection of streptozotocin in rats produces both oxidative stress in the brain and cognitive impairment. Life Sci. 2001; 68:1021–1029.
Article81. Ferreiro E, Eufrásio A, Pereira C, Oliveira CR, Rego AC. Bcl-2 overexpression protects against amyloid-beta and prion toxicity in GT1-7 neural cells. J Alzheimers Dis. 2007; 12:223–228.
Article82. Rohn TT, Vyas V, Hernandez-Estrada T, Nichol KE, Christie LA, Head E. Lack of pathology in a triple transgenic mouse model of Alzheimer's disease after overexpression of the anti-apoptotic protein Bcl-2. J Neurosci. 2008; 28:3051–3059.
Article83. Kong J, Ren G, Jia N, Wang Y, Zhang H, Zhang W, et al. Effects of nicorandil in neuroprotective activation of PI3K/AKT pathways in a cellular model of Alzheimer's disease. Eur Neurol. 2013; 70:233–241.
Article84. Kudo W, Lee HP, Smith MA, Zhu X, Matsuyama S, Lee HG. Inhibition of Bax protects neuronal cells from oligomeric Aβ neurotoxicity. Cell Death Dis. 2012; 3:e309.
Article85. Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006; 443:787–795.
Article86. Slee EA, Adrain C, Martin SJ. Serial killers: ordering caspase activation events in apoptosis. Cell Death Differ. 1999; 6:1067–1074.
Article87. Montagnani M, Chen H, Barr VA, Quon MJ. Insulin-stimulated activation of eNOS is independent of Ca2+ but requires phosphorylation by Akt at Ser(1179). J Biol Chem. 2001; 276:30392–30398.
Article88. Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol. 2001; 2:760–768.
Article89. Brunet A, Datta SR, Greenberg ME. Transcription-dependent and -independent control of neuronal survival by the PI3K-Akt signaling pathway. Curr Opin Neurobiol. 2001; 11:297–305.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Erratum to "Agmatine Improves Cognitive Dysfunction and Prevents Cell Death in a Streptozotocin-Induced Alzheimer Rat Model" by Song J, et al. (Yonsei Med J 2014;55:689-99.)
- Hyperpolarized [1- 13C]pyruvate Magnetic Resonance Spectroscopy Shows That Agmatine Increased Lactate Production in the Brain of Type 2 Diabetic Mice
- A Novel Histone Deacetylase 6 Inhibitor, 4-FHA, Improves Scopolamine-Induced Cognitive and Memory Impairment in Mice
- Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain
- The Effects of Agmatine on Apoptosis Induced by Capsaicin in Mouse Hippocampal Neuron