Yonsei Med J.
2014 May;55(3):570-575. 10.3349/ymj.2014.55.3.570.
Unrecognized Kinetics of Serum Testosterone: Impact on Short-Term Androgen Deprivation Therapy for Prostate Cancer
- Affiliations
-
- 1Department of Urology and Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea. chung646@yuhs.ac
- 2Sex Offender Treatment and Rehabilitation Center, National Forensic Hospital, Gongju, Korea.
- KMID: 2068664
- DOI: http://doi.org/10.3349/ymj.2014.55.3.570
Abstract
- PURPOSE
To evaluate the kinetics of serum testosterone (T) recovery following short-term androgen deprivation therapy (ADT), as the understanding thereof is essential for the proper management of prostate cancer (PCa), especially intermittent ADT.
MATERIALS AND METHODS
This prospective analysis included male sex offenders who voluntarily received leuprolide acetate in order to alleviate sexual aberrance. Thirty-three and 25 patients who received 3 and 6 months of ADT were assigned to Group A and Group B, respectively. Serum T levels were obtained every week during the on-cycle period, then monthly during the off-cycle period for at least 12 months.
RESULTS
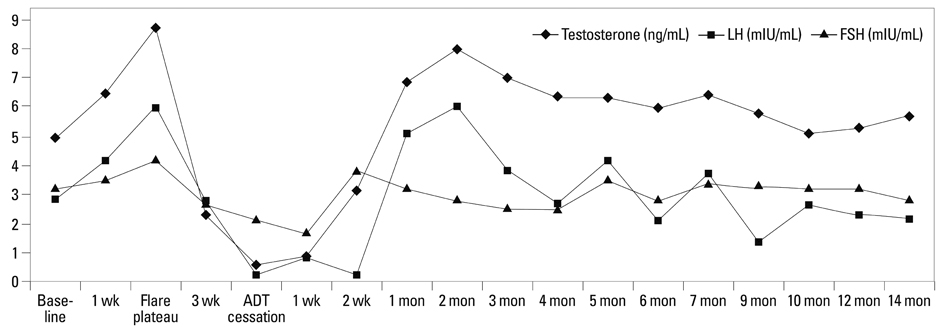
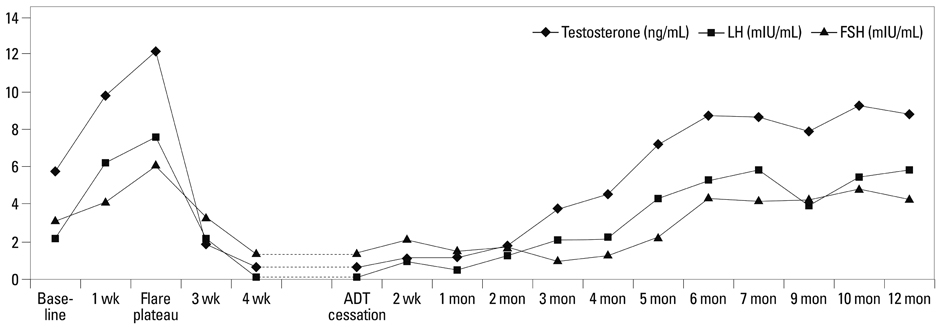
The kinetics of serum T during the on-cycle period were similar in both groups. After flare reaction at week 2, a nadir of 0.45+/-0.29 ng/mL was achieved. In Group A, an abrupt rebound-upsurge was observed during the first 2 month off-cycle period, which surpassed the baseline level and reached a plateau level of 8.74+/-2.11 ng/mL during the flare (p<0.001). This upsurge was followed by a gradual decline back to baseline over the following 10 months. In Group B, a gradual increase was observed, and a baseline level of 7.26+/-1.73 ng/mL was reached at 5 months. Thereafter, an ongoing upsurge that surpassed baseline levels was observed until 12 months (8.81+/-1.92 ng/mL; p=0.002).
CONCLUSION
The kinetics of serum T recovery during the off-cycle period varied according to the duration of ADT. Serum T should be monitored beyond normalization, as an excessive rebound may improve quality-of-life, but hamper the treatment efficacy of PCa.
Keyword
MeSH Terms
-
Adult
Androgen Antagonists/*therapeutic use
Follicle Stimulating Hormone/blood
Humans
Luteinizing Hormone/blood
Male
Middle Aged
Prospective Studies
Prostatic Neoplasms/*blood/*drug therapy
Testosterone/*blood
Treatment Outcome
Young Adult
Androgen Antagonists
Follicle Stimulating Hormone
Luteinizing Hormone
Testosterone
Figure
Cited by 2 articles
-
Exponential Rise in Prostate-Specific Antigen (PSA) during Anti-Androgen Withdrawal Predicts PSA Flare after Docetaxel Chemotherapy in Patients with Castration-Resistant Prostate Cancer
Kyung Seok Han, Sung Joon Hong
Yonsei Med J. 2015;56(2):368-374. doi: 10.3349/ymj.2015.56.2.368.Survival Outcomes of Concurrent Treatment with Docetaxel and Androgen Deprivation Therapy in Metastatic Castration-Resistant Prostate Cancer
Ho Seong Jang, Kyo Chul Koo, Kang Su Cho, Byung Ha Chung
Yonsei Med J. 2016;57(5):1070-1078. doi: 10.3349/ymj.2016.57.5.1070.
Reference
-
1. Gulley JL, Aragon-Ching JB, Steinberg SM, Hussain MH, Sartor O, Higano CS, et al. Kinetics of serum androgen normalization and factors associated with testosterone reserve after limited androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2008; 180:1432–1437.
Article2. Walsh PC, DeWeese TL, Eisenberger MA. A structured debate: immediate versus deferred androgen suppression in prostate cancer-evidence for deferred treatment. J Urol. 2001; 166:508–515.
Article3. Sciarra A, Abrahamsson PA, Brausi M, Galsky M, Mottet N, Sartor O, et al. Intermittent androgen-deprivation therapy in prostate cancer: a critical review focused on phase 3 trials. Eur Urol. 2013; 64:722–730.
Article4. NCCN clinical practice guidelines in oncology (NCCN Guideline® on prostate cancer. NCCN web site 2013. Available at: http://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf.5. Spry NA, Galvão DA, Davies R, La Bianca S, Joseph D, Davidson A, et al. Long-term effects of intermittent androgen suppression on testosterone recovery and bone mineral density: results of a 33-month observational study. BJU Int. 2009; 104:806–812.
Article6. Oefelein MG. Time to normalization of serum testosterone after 3-month luteinizing hormone-releasing hormone agonist administered in the neoadjuvant setting: implications for dosing schedule and neoadjuvant study consideration. J Urol. 1998; 160:1685–1688.
Article7. Nejat RJ, Rashid HH, Bagiella E, Katz AE, Benson MC. A prospective analysis of time to normalization of serum testosterone after withdrawal of androgen deprivation therapy. J Urol. 2000; 164:1891–1894.
Article8. Teillac P, Bono AV, Irani J, Wirth MP, Zlotta AR. The role of luteinizing hormone-releasing hormone therapy in locally advanced prostate cancer and biochemical failure: considerations for optimal use. Clin Ther. 2005; 27:273–285.
Article9. Knudsen KE, Penning TM. Partners in crime: deregulation of AR activity and androgen synthesis in prostate cancer. Trends Endocrinol Metab. 2010; 21:315–324.
Article10. Pathak AS, Pacificar JS, Shapiro CE, Williams SG. Determining dosing intervals for luteinizing hormone releasing hormone agonists based on serum testosterone levels: a prospective study. J Urol. 2007; 177:2132–2135.
Article11. Koo KC, Ahn JH, Hong SJ, Lee JW, Chung BH. Effects of Chemical Castration on Sex Offenders in Relation to the Kinetics of Serum Testosterone Recovery: Implications for Dosing Schedule. J Sex Med. 2014; [Epub ahead of print].
Article12. Hill A, Briken P, Kraus C, Strohm K, Berner W. Differential pharmacological treatment of paraphilias and sex offenders. Int J Offender Ther Comp Criminol. 2003; 47:407–421.
Article13. Oefelein MG, Feng A, Scolieri MJ, Ricchiutti D, Resnick MI. Reassessment of the definition of castrate levels of testosterone: implications for clinical decision making. Urology. 2000; 56:1021–1024.
Article14. Tsai HT, Penson DF, Makambi KH, Lynch JH, Van Den Eeden SK, Potosky AL. Efficacy of intermittent androgen deprivation therapy vs conventional continuous androgen deprivation therapy for advanced prostate cancer: a meta-analysis. Urology. 2013; 82:327–333.
Article15. Bruchovsky N, Klotz L, Crook J, Goldenberg SL. Locally advanced prostate cancer--biochemical results from a prospective phase II study of intermittent androgen suppression for men with evidence of prostate-specific antigen recurrence after radiotherapy. Cancer. 2007; 109:858–867.
Article16. Pickles T, Agranovich A, Berthelet E, Duncan GG, Keyes M, Kwan W, et al. Testosterone recovery following prolonged adjuvant androgen ablation for prostate carcinoma. Cancer. 2002; 94:362–367.
Article17. Hall MC, Fritzsch RJ, Sagalowsky AI, Ahrens A, Petty B, Roehrborn CG. Prospective determination of the hormonal response after cessation of luteinizing hormone-releasing hormone agonist treatment in patients with prostate cancer. Urology. 1999; 53:898–902.
Article18. Sethi R, Sanfilippo N. Six-month depot formulation of leuprorelin acetate in the treatment of prostate cancer. Clin Interv Aging. 2009; 4:259–267.19. Gulley JL, Figg WD, Steinberg SM, Carter J, Sartor O, Higano CS, et al. A prospective analysis of the time to normalization of serum androgens following 6 months of androgen deprivation therapy in patients on a randomized phase III clinical trial using limited hormonal therapy. J Urol. 2005; 173:1567–1571.
Article20. Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, et al. The influence of finasteride on the development of prostate cancer. N Engl J Med. 2003; 349:215–224.
Article21. Roehrborn CG, Nickel JC, Andriole GL, Gagnier RP, Black L, Wilson TH, et al. Dutasteride improves outcomes of benign prostatic hyperplasia when evaluated for prostate cancer risk reduction: secondary analysis of the REduction by DUtasteride of prostate Cancer Events (REDUCE) trial. Urology. 2011; 78:641–646.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Concepts in Androgen Deprivation Therapy
- Role of Androgen Receptor in Prostate Cancer: A Review
- Chemotherapy With Androgen Deprivation for Hormone-Naïve Prostate Cancer
- Intermittent Androgen Deprivation with Goserelin and Flutamide for Prostate Cancer: a Pilot Study
- Long-Lasting Antiandrogen Withdrawal Syndrome in Castration-Resistant Prostate Cancer: Three Cases With Complete Response