J Korean Neurosurg Soc.
2015 Jul;58(1):30-35. 10.3340/jkns.2015.58.1.30.
Clinical Neuropathological Analysis of 10 Cases of Cerebral Amyloid Angiopathy-Related Cerebral Lobar Hemorrhage
- Affiliations
-
- 1Department of Neurology, The General Hospital of Shenyang Military Region, Shenyang, China. xiaoqiulicn@163.com
- 2Department of Neurology, 463th Hospital of Chinese People's Liberation Army, Shenyang, China.
- KMID: 2067101
- DOI: http://doi.org/10.3340/jkns.2015.58.1.30
Abstract
OBJECTIVE
The clinical and pathological characteristics of 10 cases of cerebral amyloid angiopathy (CAA)-related cerebral lobar hemorrhage (CLH) that was diagnosed at autopsy were investigated to facilitate the diagnosis of this condition.
METHODS
The clinical characteristics of 10 cases of CAA-related CLH were retrospectively reviewed, and a neuropathological examination was performed on autopsy samples.
RESULTS
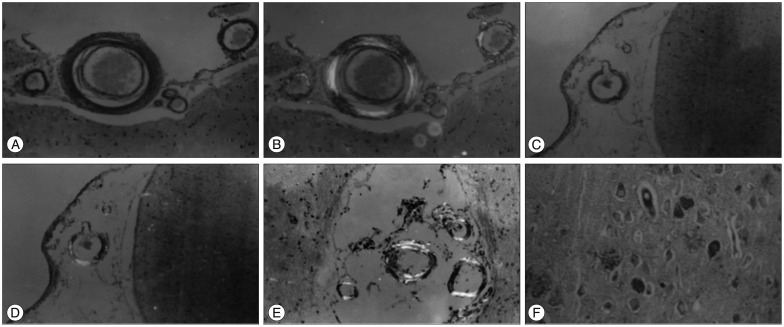
The 10 cases included two with a single lobar hemorrhage and eight with multifocal lobar hemorrhages. In all of the cases, the hemorrhage bled into the subarachnoid space. Pathological examinations of the 10 cases revealed microaneurysms in two, double barrel-like changes in four, multifocal arteriolar clusters in five, obliterative onion skin-like intimal changes in four, fibrinoid necrosis of the vessels in seven, neurofibrillary tangles in eight, and senile plaques in five cases.
CONCLUSION
CAA-related CLHs were located primarily in the parietal, temporal, and occipital lobes. These hemorrhages normally consisted of multiple repeated CLHs that frequently bled into the subarachnoid space. CAA-associated microvascular lesions may be the pathological factor underlying CLH.
MeSH Terms
Figure
Cited by 1 articles
-
Cerebral Arterial Stenosis in Patients with Spontaneous Intracerebral Hemorrhage
Pil-Wook Chung, Yu Sam Won
J Korean Neurosurg Soc. 2017;60(5):511-517. doi: 10.3340/jkns.2016.1011.003.
Reference
-
1. Alexander M, Patil AK, Mathew V, Sivadasan A, Chacko G, Mani SE. Recurrent craniospinal subarachnoid hemorrhage in cerebral amyloid angiopathy. Ann Indian Acad Neurol. 2013; 16:97–99. PMID: 23661974.
Article2. Antonell A, Gelpi E, Sánchez-Valle R, Martínez R, Molinuevo JL, Lladó A. Breakpoint sequence analysis of an AβPP locus duplication associated with autosomal dominant Alzheimer's disease and severe cerebral amyloid angiopathy. J Alzheimers Dis. 2012; 28:303–308. PMID: 22008262.
Article3. Bu G. Apolipoprotein E and its receptors in Alzheimer's disease : pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009; 10:333–344. PMID: 19339974.
Article4. Chao CP, Kotsenas AL, Broderick DF. Cerebral amyloid angiopathy : CT and MR imaging findings. Radiographics. 2006; 26:1517–1531. PMID: 16973779.
Article5. Charidimou A, Gang Q, Werring DJ. Sporadic cerebral amyloid angiopathy revisited : recent insights into pathophysiology and clinical spectrum. J Neurol Neurosurg Psychiatry. 2012; 83:124–137. PMID: 22056963.
Article6. Charidimou A, Jäger HR, Werring DJ. Cerebral microbleed detection and mapping : principles, methodological aspects and rationale in vascular dementia. Exp Gerontol. 2012; 47:843–852. PMID: 22750456.
Article7. Chouraki A, Rollin-Sillaire A, Deramecourt V, Zairi F, Le Rhun E, Cordonnier C, et al. Cerebral amyloid angiopathy revealed by rapidly progressing leptomeningeal lesions. J Neurol. 2014; 261:1432–1435. PMID: 24859330.
Article8. Eisele YS, Fritschi SK, Hamaguchi T, Obermüller U, Füger P, Skodras A, et al. Multiple factors contribute to the peripheral induction of cerebral β-amyloidosis. J Neurosci. 2014; 34:10264–10273. PMID: 25080588.
Article9. Gahr M, Nowak DA, Connemann BJ, Schönfeldt-Lecuona C. Cerebral amyloidal angiopathy--a disease with implications for neurology and psychiatry. Brain Res. 2013; 1519:19–30. PMID: 23651976.
Article10. Greenberg SM, Al-Shahi Salman R, Biessels GJ, van Buchem M, Cordonnier C, Lee JM, et al. Outcome markers for clinical trials in cerebral amyloid angiopathy. Lancet Neurol. 2014; 13:419–428. PMID: 24581702.
Article11. Hawkes CA, Jayakody N, Johnston DA, Bechmann I, Carare RO. Failure of perivascular drainage of β-amyloid in cerebral amyloid angiopathy. Brain Pathol. 2014; 24:396–403. PMID: 24946077.
Article12. Hirohata M, Yoshita M, Ishida C, Ikeda SI, Tamaoka A, Kuzuhara S, et al. Clinical features of non-hypertensive lobar intracerebral hemorrhage related to cerebral amyloid angiopathy. Eur J Neurol. 2010; 17:823–829. PMID: 20158508.
Article13. Illsley A, Ramadan H. Cerebral amyloid angiopathy : a transient ischaemic attack mimic. Clin Med. 2014; 14:255–259. PMID: 24889568.
Article14. Itoh Y, Yamada M. Cerebral amyloid angiopathy in the elderly : the clinicopathological features, pathogenesis, and risk factors. J Med Dent Sci. 1997; 44:11–19. PMID: 9385038.15. Johnson KA, Gregas M, Becker JA, Kinnecom C, Salat DH, Moran EK, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007; 62:229–234. PMID: 17683091.
Article16. Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy : validation of the Boston criteria. Neurology. 2001; 56:537–539. PMID: 11222803.
Article17. Labovitz DL, Halim A, Boden-Albala B, Hauser WA, Sacco RL. The incidence of deep and lobar intracerebral hemorrhage in whites, blacks, and Hispanics. Neurology. 2005; 65:518–522. PMID: 16116109.
Article18. Linn J, Halpin A, Demaerel P, Ruhland J, Giese AD, Dichgans M, et al. Prevalence of superficial siderosis in patients with cerebral amyloid angiopathy. Neurology. 2010; 74:1346–1350. PMID: 20421578.
Article19. Maeda A, Yamada M, Itoh Y, Otomo E, Hayakawa M, Miyatake T. Computer-assisted three-dimensional image analysis of cerebral amyloid angiopathy. Stroke. 1993; 24:1857–1864. PMID: 8248968.
Article20. Martucci M, Sarria S, Toledo M, Coscojuela P, Vert C, Siurana S, et al. Cerebral amyloid angiopathy-related inflammation : imaging findings and clinical outcome. Neuroradiology. 2014; 56:283–289. PMID: 24493378.
Article21. McCarron MO, Nicoll JA, Ironside JW, Love S, Alberts MJ, Bone I. Cerebral amyloid angiopathy-related hemorrhage. Interaction of APOE epsilon2 with putative clinical risk factors. Stroke. 1999; 30:1643–1646. PMID: 10436115.22. Mehndiratta P, Manjila S, Ostergard T, Eisele S, Cohen ML, Sila C, et al. Cerebral amyloid angiopathy-associated intracerebral hemorrhage : pathology and management. Neurosurg Focus. 2012; 32:E7. PMID: 22463117.23. Mendel TA, Wierzba-Bobrowicz T, Lewandowska E, Stępień T, Szpak GM. The development of cerebral amyloid angiopathy in cerebral vessels. A review with illustrations based upon own investigated post mortem cases. Pol J Pathol. 2013; 64:260–267. PMID: 24375040.
Article24. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005; 365:387–397. PMID: 15680453.
Article25. Nicoll JA, Burnett C, Love S, Graham DI, Dewar D, Ironside JW, et al. High frequency of apolipoprotein E epsilon 2 allele in hemorrhage due to cerebral amyloid angiopathy. Ann Neurol. 1997; 41:716–721. PMID: 9189032.
Article26. O'Donnell HC, Rosand J, Knudsen KA, Furie KL, Segal AZ, Chiu RI, et al. Apolipoprotein E genotype and the risk of recurrent lobar intracerebral hemorrhage. N Engl J Med. 2000; 342:240–245. PMID: 10648765.27. Qureshi AI, Mendelow AD, Hanley DF. Intracerebral haemorrhage. Lancet. 2009; 373:1632–1644. PMID: 19427958.
Article28. Qureshi AI, Tuhrim S, Broderick JP, Batjer HH, Hondo H, Hanley DF. Spontaneous intracerebral hemorrhage. N Engl J Med. 2001; 344:1450–1460. PMID: 11346811.
Article29. Rensink AA, de Waal RM, Kremer B, Verbeek MM. Pathogenesis of cerebral amyloid angiopathy. Brain Res Brain Res Rev. 2003; 43:207–223. PMID: 14572915.
Article30. Reuter B, Grudzenski S, Chatzikonstantinou E, Meairs S, Ebert A, Heiler P, et al. Thrombolysis in experimental cerebral amyloid angiopathy and the risk of secondary intracerebral hemorrhage. Stroke. 2014; 45:2411–2416. PMID: 25005438.
Article31. Revesz T, Ghiso J, Lashley T, Plant G, Rostagno A, Frangione B, et al. Cerebral amyloid angiopathies : a pathologic, biochemical, and genetic view. J Neuropathol Exp Neurol. 2003; 62:885–898. PMID: 14533778.
Article32. Roher AE, Cribbs DH, Kim RC, Maarouf CL, Whiteside CM, Kokjohn TA, et al. Bapineuzumab alters aβ composition : implications for the amyloid cascade hypothesis and anti-amyloid immunotherapy. PLoS One. 2013; 8:e59735. PMID: 23555764.33. Rosand J, Muzikansky A, Kumar A, Wisco JJ, Smith EE, Betensky RA, et al. Spatial clustering of hemorrhages in probable cerebral amyloid angiopathy. Ann Neurol. 2005; 58:459–462. PMID: 16130107.
Article34. Sakurai K, Tokumaru AM, Nakatsuka T, Murayama S, Hasebe S, Imabayashi E, et al. Imaging spectrum of sporadic cerebral amyloid angiopathy : multifaceted features of a single pathological condition. Insights Imaging. 2014; 5:375–385. PMID: 24519790.
Article35. Sudlow CL, Warlow CP. Comparable studies of the incidence of stroke and its pathological types : results from an international collaboration. International Stroke Incidence Collaboration. Stroke. 1997; 28:491–499. PMID: 9056601.
Article36. Tang YJ, Li Y, Wang S, Zhu MW, Sun YL, Zhao JZ. The incidence of cerebral amyloid angiopathy in surgically treated intracranial hemorrhage in the Chinese population. Neurosurg Rev. 2013; 36:533–539. PMID: 23765214.
Article37. Tomimoto H. Subcortical vascular dementia. Neurosci Res. 2011; 71:193–199. PMID: 21821070.
Article38. Yamada M. Brain hemorrhages in cerebral amyloid angiopathy. Semin Thromb Hemost. 2013; 39:955–962. PMID: 24108472.
Article39. Yamada M, Naiki H. Cerebral amyloid angiopathy. Prog Mol Biol Transl Sci. 2012; 107:41–78. PMID: 22482447.
Article40. Ye C, Ruan Y, Zou L, Li G, Li C, Chen Y, et al. The 2011 survey on hypertensive disorders of pregnancy (HDP) in China : prevalence, risk factors, complications, pregnancy and perinatal outcomes. PLoS One. 2014; 9:e100180. PMID: 24937406.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cerebral Amyloid Angiopathy-related Intracerebral Hemorrhage
- Lobar Intracerebral Hemorrhage Associated With Cortical Superficial Siderosis
- Multiple Recurrent Cerebral Hemorrhages Related to Cerebral Amyloid Angiopathy with Arterial Hypertension
- Cerebral Amyloid Angiopathy: An Undeniable Small Vessel Disease
- Concomitant Small Intracerebral Hemorrhage in a Patient with Cerebral Amyloid Angiopathy Mimicking Transient Ischemic Attack