Korean J Urol.
2007 Aug;48(8):797-803. 10.4111/kju.2007.48.8.797.
A Study on the Incidence and Preoperative Predicting Factors of Extraprostatic Extension in T1c Prostate Cancers
- Affiliations
-
- 1Department of Urology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. selee@snubh.org
- KMID: 2061272
- DOI: http://doi.org/10.4111/kju.2007.48.8.797
Abstract
- PURPOSE
To evaluate the incidence and identify the predicting factors of extraprostatic extension(EPE) in T1c prostate cancers.
MATERIALS AND METHODS
Of 267 consecutive men who underwent radical retropubic prostatectomy(RRP) as initial treatment for prostate cancers, 131(49.1%) presented with a clinical stage T1c disease. Clinicopathological data were collected, and factors related to biopsy collected; i.e. the number of positive cores(No.(+) core); the percentage of positive cores(%(+) core); the maximal tumor length(Max. mm cancer); the sum of tumor length (Total mm cancer); the maximal ratio of tumor/core length(Max. % mm cancer) and the mean ratio of tumor/core length(Mean % mm cancer). A logistical regression analysis was performed after dividing the cases into organ-confined(OC) and EPE.
RESULTS
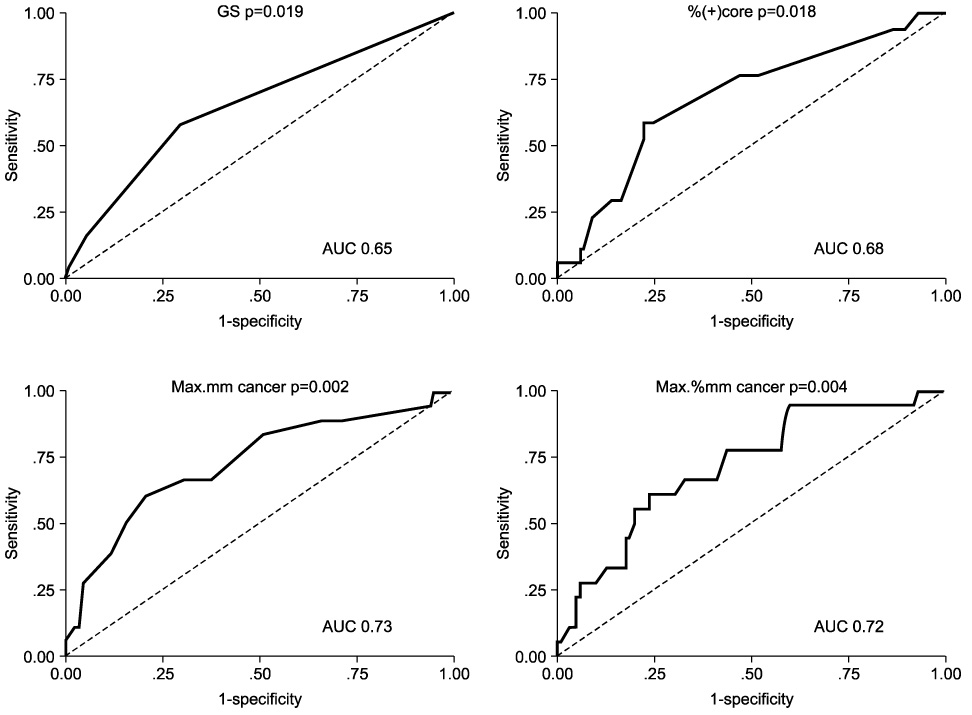
Of the T1c tumors, 107(81.7%) and 24(18.3%) were found to be OC and to have EPE after RRP, respectively. The preoperative factors that showed a significant difference between the two groups(OC vs. EPE) were %free prostate-specific antigen(17.7 vs. 11.1%), prostate volume(43.5 vs. 34.6ml), Gleason score(6.4 vs. 6.8), %(+) core(17.9 vs. 27%), Max. mm cancer(3.5 vs. 6.7mm) and Max. % mm cancer(24.0 vs. 41.6%). Of these factors, those significantly predicting EPE in the receiver operator characteristics curve were: the Gleason score, %(+) core, Max. mm cancer and Max. % mm cancer. Of these, only the %(+) core and Max. mm cancer were significant in predicting EPE in the multivariate logistical regression. When the cutoff of %(+) core was 19%, the risk of EPE increased 2.3 times, and when the cutoff of Max. mm cancer was 5mm the risk increased 3.6 times.
CONCLUSIONS
Max. mm cancer and %(+) core during a biopsy are preoperative factors that predict the EPE of a clinical stage T1c disease, and should be considered for modifying the surgical technique and in establishing treatment plans.
Figure
Reference
-
1. Cooperberg MR, Lubeck DP, Meng MV, Mehta SS, Carroll PR. The changing face of low-risk prostate cancer: trends in clinical presentation and primary management. J Clin Oncol. 2004. 22:2141–2149.2. Scaletscky R, Koch MO, Eckstein CW, Bicknell SL, Gray GF, Smith JA. Tumor volume and stage in carcinoma of the prostate detected by elevations in prostate specific antigen. J Urol. 1994. 152:129–131.3. Carter HB, Sauvageot J, Walsh PC, Epstein JI. Prospective evaluation of men with stage T1c adenocarcinoma of the prostate. J Urol. 1997. 157:2206–2209.4. Epstein JI, Pizov G, Walsh PC. Correlation of pathologic findings with progression after radical retropubic prostatectomy. Cancer. 1993. 71:3582–3593.5. Epstein JI, Walsh PC, Carmichael M, Brendler CB. Pathologic and clinical findings to predict tumor extent of nonpalpable (stage T1c) prostate cancer. JAMA. 1994. 271:368–374.6. Song C, Kang T, Lee M, Ro JY, Lee SE, Lee E, et al. Clinico-pathological characteristics of prostate cancer in Korean men and nomograms for the prediction of the pathological stage of the clinically localized prostate cancer: a multi-institutional update. Korean J Urol. 2007. 48:125–130.7. Freedland SJ, Mangold LA, Walsh PC, Partin AW. The prostatic specific antigen era is alive and well: prostatic specific antigen and biochemical progression following radical prostatectomy. J Urol. 2005. 174:1276–1281.8. Han M, Partin AW, Pound CR, Epstein JI, Walsh PC. Long-term biochemical disease-free and cancer-specific survival following anatomic radical retropubic prostatectomy. The 15-year Johns Hopkins experience. Urol Clin North Am. 2001. 28:555–565.9. Ghavamian R, Blute ML, Bergstralh EJ, Slezak J, Zincke H. Comparison of clinically nonpalpable prostate-specific antigen-detected (cT1c) versus palpable (cT2) prostate cancers in patients undergoing radical retropubic prostatectomy. Urology. 1999. 54:105–110.10. Stamey TA, Donaldson AN, Yemoto CE, McNeal JE, Sozen S, Gill H. Histological and clinical findings in 896 consecutive prostates treated only with radical retropubic prostatectomy: epidemiologic significance of annual changes. J Urol. 1998. 160:2412–2417.11. Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982. 128:492–497.12. Stolzenburg JU, Rabenalt R, Tannapfel A, Liatsikos EN. Intrafascial nerve-sparing endoscopic extraperitoneal radical prostatectomy. Urology. 2006. 67:17–21.13. Cho HS, Kim CS, Ahn H. The pathological characteristics of localized prostate cancer, managed with radical prostatectomy. Korean J Urol. 2002. 43:938–943.14. Park HK, Hong SK, Byun SS, Lee SE. Comparison of the rate of detecting prostate cancer and the pathologic characteristics of the patients with a serum PSA level in the range of 3.0 to 4.0ng/ml and the patients with a serum PSA level in the range 4.1 to 10.0ng/ml. Korean J Urol. 2006. 47:358–361.15. Elgamal AA, Van Poppel HP, Van de Voorde WM, Van Dorpe JA, Oyen RH, Baert LV. Impalpable invisible stage T1c prostate cancer: characteristics and clinical relevance in 100 radical prostatectomy specimens--a different view. J Urol. 1997. 157:244–250.16. Veltri RW, Miller MC, Mangold LA, O'Dowd GJ, Epstein JI, Partin AW. Prediction of pathological stage in patients with clinical stage T1c prostate cancer: the new challenge. J Urol. 2002. 168:100–104.17. Armatys SA, Koch MO, Bihrle R, Gardner TA, Cheng L. Is it necessary to separate clinical stage T1c from T2 prostate adenocarcinoma? BJU Int. 2005. 96:777–780.18. Catalona WJ, Richie JP, Ahmann FR, Hudson MA, Scardino PT, Flanigan RC, et al. Comparison of digital rectal examination and serum prostate specific antigen in the early detection of prostate cancer: results of a multicenter clinical trial of 6,630 men. J Urol. 1994. 151:1283–1290.19. Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997. 277:1445–1451.20. Ravery V, Chastang C, Toublanc M, Boccon-Gibod L, Delmas V, Boccon-Gibod L. Percentage of cancer on biopsy cores accurately predicts extracapsular extension and biochemical relapse after radical prostatectomy for T1-T2 prostate cancer. Eur Urol. 2000. 37:449–455.21. Freedland SJ, Csathy GS, Dorey F, Aronson WJ. Percent prostate needle biopsy tissue with cancer is more predictive of biochemical failure or adverse pathology after radical prostatectomy than prostate specific antigen or Gleason score. J Urol. 2002. 167:516–520.22. San Francisco IF, Regan MM, Olumi AF, DeWolf WC. Percent of cores positive for cancer is a better preoperative predictor of cancer recurrence after radical prostatectomy than prostate specific antigen. J Urol. 2004. 171:1492–1499.23. Poulos CK, Daggy JK, Cheng L. Prostate needle biopsies: multiple variables are predictive of final tumor volume in radical prostatectomy specimens. Cancer. 2004. 101:527–532.24. Naya Y, Slaton JW, Troncoso P, Okihara K, Babaian RJ. Tumor length and location of cancer on biopsy predict for side specific extraprostatic cancer extension. J Urol. 2004. 171:1093–1097.25. Park SW, Park JY, Ahn H. Ability of core biopsies to predict extracapsular extension of prostate cancer. Korean J Urol. 2004. 45:647–652.26. Ogawa O, Egawa S, Arai Y, Tobisu K, Yoshida O, Kato T. Preoperative predictors for organ-confined disease in Japanese patients with stage T1c prostate cancer. Int J Urol. 1998. 5:454–458.27. Ou YC, Chen JT, Yang CR, Cheng CL, Ho HC, Kao YL, et al. Preoperative prediction of extracapsular tumor extension at radical retropubic prostatectomy in Taiwanese patients with T1c prostate cancer. Jpn J Clin Oncol. 2002. 32:172–176.28. Southwick PC, Catalona WJ, Partin AW, Slawin KM, Brawer MK, Flanigan RC, et al. Prediction of post-radical prostatectomy pathological outcome for stage T1c prostate cancer with percent free prostate specific antigen: a prospective multicenter clinical trial. J Urol. 1999. 162:1346–1351.29. Makarov DV, Humphreys EB, Mangold LA, Walsh PC, Partin AW, Epstein JI, et al. Pathological outcomes and biochemical progression in men with T1c prostate cancer undergoing radical prostatectomy with prostate specific antigen 2.6 to 4.0 vs 4.1 to 6.0ng/ml. J Urol. 2006. 176:554–558.30. Kim YJ, Chang IH, Gil MC, Hong SK, Byun SS, Lee SE. Concordance of Gleason scores between prostate needle biopsy and radical prostatectomy specimens according to the number of biopsy cores. Korean J Urol. 2006. 47:482–488.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Usefulness of Preoperative Serum Testosterone as a Predictor of Extraprostatic Extension and Biochemical Recurrence
- The Role of Endorectal Magnetic Resonance Imaging in Predicting Extraprostatic Extension and Seminal Vesicle Invasion in Clinically Localized Prostate Cancer
- Development of Nomogram for Predicting Pathologic Outcome using Prostate-specific Antigen, Gleason Score, and the Percentage of Positive Core in the Clinically Confined Prostate Cancers, and Comparison with Nomogram using Existing Factors
- Preoperative Prostatic Biopsy Factors for the Prediction of Pathologic Stage after Radical Prostatectomy
- The Anatomic Distribution and Pathological Characteristics of Prostate Cancer: A Mapping Analysis