Korean J Urol.
2007 Aug;48(8):789-796. 10.4111/kju.2007.48.8.789.
Development of Nomogram for Predicting Pathologic Outcome using Prostate-specific Antigen, Gleason Score, and the Percentage of Positive Core in the Clinically Confined Prostate Cancers, and Comparison with Nomogram using Existing Factors
- Affiliations
-
- 1Department of Urology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. selee@snubh.org
- KMID: 2061271
- DOI: http://doi.org/10.4111/kju.2007.48.8.789
Abstract
- PURPOSE
There have been reports that clinical stages do not reflect patients' postoperative prognosis well. On the contrary, the clinical application of the percentage of positive core(%(+) core), which predicts tumor volume has been increasing. We developed nomogram for predicting pathologic outcome using prostate-specific antigen(PSA), Gleason score and %(+) core based on data of radical prostatectomy and compared it with nomogram using clinical stage instead of %(+) core.
MATERIALS AND METHODS
Two hundred and fifty nine patients with clinically confined prostate cancers were included in the study. Nomogram for predicting pathologic outcome was developed through multinominal logistic regression analysis, and pathologic outcomes were extracapsular invasion(ECE), seminal vesicle invasion(SVI) and bladder neck invasion(BNI). The accuracy of each nomogram for predicting each pathologic outcome was compared on the basis of receiver operating characteristic(ROC) curve analysis.
RESULTS
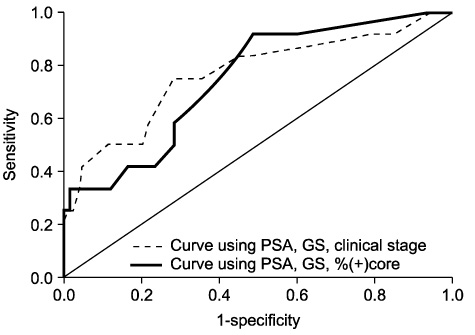
The mean %(+) core was 24.6% and clinical stages T1c, T2a,b and T2c were 58.7%, 32.0% and 9.3%, respectively. ECE was observed in 45(17.4%), SVI in 9(3.5%), and BNI in 12(4.6%). With an increase in PSA, Gleason score, clinical stage, or %(+) core, the incidence of extraprostatic involvement increased gradually. Two nomograms for predicting pathologic outcome were developed. In quantifying expected predictive improvement, area under ROC curve for predicting ECE was greater in the nomogram using %(+) core than clinical stage(0.815 vs. 0.778). These values for predicting SVI were 0.886 and 0.760, respectively, and for predicting BNI, 0.743 and 0.764, respectively.
CONCLUSIONS
We developed nomogram for predicting pathologic outcomes using %(+) core instead of clinical stage. Nomogram using %(+) core predicted ECE and SVI with greater accuracy than nomogram using clinical stage.
Keyword
MeSH Terms
Figure
Reference
-
1. Pound CR, Partin AW, Epstein JI, Walsh PC. Prostate-specific antigen after anatomic radical retropubic prostatectomy. Patterns of recurrence and cancer control. Urol Clin North Am. 1997. 24:395–406.2. Partin AW, Yoo J, Carter HB, Pearson JD, Chan DW, Epstein JI, et al. The use of prostate specific antigen, clinical stage and Gleason score to predict pathological stage in men with localized prostate cancer. J Urol. 1993. 150:110–114.3. Partin AW, Kattan MW, Subong EN, Walsh PC, Wojno KJ, Oesterling JE, et al. Combination of prostate-specific antigen, clinical stage, and Gleason score to predict pathological stage of localized prostate cancer. A multi-institutional update. JAMA. 1997. 277:1445–1451.4. Partin AW, Mangold LA, Lamm DM, Walsh PC, Epstein JI, Pearson JD. Contemporary update of prostate cancer staging nomograms (Partin Tables) for the new millennium. Urology. 2001. 58:843–848.5. Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. J Natl Cancer Inst. 1998. 90:766–771.6. Han M, Snow PB, Brandt JM, Partin AW. Evaluation of artificial neural networks for the prediction of pathologic stage in prostate carcinoma. Cancer. 2001. 91:1661–1666.7. Chun FK, Briganti A, Gallina A, Hutterer GC, Shariat SF, Antebie E, et al. Prostate-specific antigen improves the ability of clinical stage and biopsy Gleason sum to predict the pathologic stage at radical prostatectomy in the new millennium. Eur Urol. 2007. [Epub ahead of print].8. Prezioso D, Galasso R, Di Martino M, Iapicca G, Iacono F. Role of surgery in treatment of locally advanced prostate cancer. Anticancer Res. 2006. 26:3151–3158.9. Rioux-Leclercq NC, Chan DY, Epstein JI. Prediction of outcome after radical prostatectomy in men with organ-confined Gleason score 8 to 10 adenocarcinoma. Urology. 2002. 60:666–669.10. Hull GW, Rabbani F, Abbas F, Wheeler TM, Kattan MW, Scardino PT. Cancer control with radical prostatectomy alone in 1,000 consecutive patients. J Urol. 2002. 167:528–534.11. Sebo TJ, Bock BJ, Cheville JC, Lohse C, Wollan P, Zincke H. The percent of cores positive for cancer in prostate needle biopsy specimens is strongly predictive of tumor stage and volume at radical prostatectomy. J Urol. 2000. 163:174–178.12. Cagiannos I, Karakiewicz P, Eastham JA, Ohori M, Rabbani F, Gerigk C, et al. A preoperative nomogram identifying decreased risk of positive pelvic lymph nodes in patients with prostate cancer. J Urol. 2003. 170:1798–1803.13. Egawa S, Suyama K, Arai Y, Matsumoto K, Tsukayama C, Kuwao S, et al. A study of pretreatment nomograms to predict pathological stage and biochemical recurrence after radical prostatectomy for clinically resectable prostate cancer in Japanese men. Jpn J Clin Oncol. 2001. 31:74–81.14. Walsh PC, Donker PJ. Impotence following radical prostatectomy: insight into etiology and prevention. J Urol. 1982. 128:492–497.15. Ward JF, Slezak JM, Blute ML, Bergstralh EJ, Zincke H. Radical prostatectomy for clinically advanced (cT3) prostate cancer since the advent of prostate-specific antigen testing: 15-year outcome. BJU Int. 2005. 95:751–756.16. Rioux-Leclercq NC, Chan DY, Epstein JI. Prediction of outcome after radical prostatectomy in men with organ-confined Gleason score 8 to 10 adenocarcinoma. Urology. 2002. 60:666–669.17. Nelson BA, Shappell SB, Chang SS, Wells N, Farnham SB, Smith JA, et al. Tumour volume is an independent predictor of prostate-specific antigen recurrence in patients undergoing radical prostatectomy for clinically localized prostate cancer. BJU Int. 2006. 97:1169–1172.18. Rubin MA, Mucci NR, Manley S, Sanda M, Cushenberry E, Strawderman M, et al. Predictors of Gleason pattern 4/5 prostate cancer on prostatectomy specimens: can high grade tumor be predicted preoperatively? J Urol. 2001. 165:114–118.19. D'Amico AV, Whittington R, Malkowicz SB, Schultz D, Fondurulia J, Chen MH, et al. Clinical utility of the percentage of positive prostate biopsies in defining biochemical outcome after radical prostatectomy for patients with clinically localized prostate cancer. J Clin Oncol. 2000. 18:1164–1172.20. Ravery V, Chastang C, Toublanc M, Boccon-Gibod L, Delmas V, Boccon-Gibod L. Percentage of cancer on biopsy cores accurately predicts extracapsular extension and biochemical relapse after radical prostatectomy for T1-T2 prostate cancer. Eur Urol. 2000. 37:449–455.21. Ross PL, Scardino PT, Kattan MW. A catalog of prostate cancer nomograms. J Urol. 2001. 165:1562–1568.22. Chun FK, Karakiewicz PI, Huland H, Graefen M. Role of nomograms for prostate cancer in 2007. World J Urol. 2007. 25:131–142.23. Song C, Kim J, Chung H, Kim CS, Ro JY, Ahn HJ. Nomograms for the prediction of the pathological stage of the clinically localized prostate cancer in Korean men. Korean J Urol. 2003. 44:753–758.24. Song C, Kang T, Lee M, Ro JY, Lee SE, Lee E, et al. Clinico-pathological characteristics of prostate cancer in Korean men and nomograms for the prediction of the pathological stage of the clinically localized prostate cancer: a multi-institutional update. Korean J Urol. 2007. 48:125–130.25. Freedland SJ, Mangold LA, Walsh PC, Partin AW. The prostatic specific antigen era is alive and well: prostatic specific antigen and biochemical progression following radical prostatectomy. J Urol. 2005. 174:1276–1281.26. Park HK, Hong SK, Byun SS, Lee SE. Comparison of the rate of detecting prostate cancer and the pathologic characteristics of the patients with a serum PSA level in the range of 3.0 to 4.0ng/ml and the patients with a serum PSA level in the range 4.1 to 10.0ng/ml. Korean J Urol. 2006. 47:358–361.27. Yossepowitch O, Sircar K, Scardino PT, Ohori M, Kattan MW, Wheeler TM, et al. Bladder neck involvement in pathological stage pT4 radical prostatectomy specimens is not an independent prognostic factor. J Urol. 2002. 168:2011–2015.28. Kim YJ, Chang IH, Gil MC, Hong SK, Byun SS, Lee SE. Concordance of Gleason scores between prostate needle biopsy and radical prostatectomy specimens according to the number of biopsy cores. Korean J Urol. 2006. 47:482–488.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy of Radical Retropubic Prostatectomy in Patients with Clinically Localized Prostate Cancer and a Biopsy Gleason Score of 8 or Higher
- Clinical Significance of a Single-Core Positive Prostate Cancers Detected on Extended Prostate Needle Biopsy
- The Value of Prostate Specific Antigen, Digital Rectal Examination, Transrectal Ultrasound, and Transrectal Ultrasound-Guided Biopsy in Prediction of Final Pathologic Diagnosis in Prostate Cancer
- Efficacy of Radical Retropubic Prostatectomy as the Primary Treatment for Patients with Clinically Localized Prostate Cancer and a Serum PSA Level >or=20ng/ml
- Nomogram for Prediction of Prostate Cancer with Serum Prostate Specific Antigen Less than 10 ng/mL