Korean J Urol.
2007 May;48(5):483-488. 10.4111/kju.2007.48.5.483.
Analysis of the NF-kappaB and Apoptosis Inducing Genes in Bladder Tumor
- Affiliations
-
- 1Department of Urology, Chungbuk National University College of Medicine, Cheongju, Korea. wjkim@chungbuk.ac.kr
- 2Department of Urology, School of Medicine, Kyung Hee University, Seoul, Korea.
- KMID: 2061251
- DOI: http://doi.org/10.4111/kju.2007.48.5.483
Abstract
-
PURPOSE: A multi-subunit transcription factor NF-kappaB mediates the antiapoptotic signals in several cancer cell lines and it is activated in a broad range of human tumors. In this study, we investigated whether the expression levels of the NF-kappaB and the apoptosis inducing genes were related to the pathogenesis and clinical properties of human bladder tumor.
MATERIALS AND METHODS
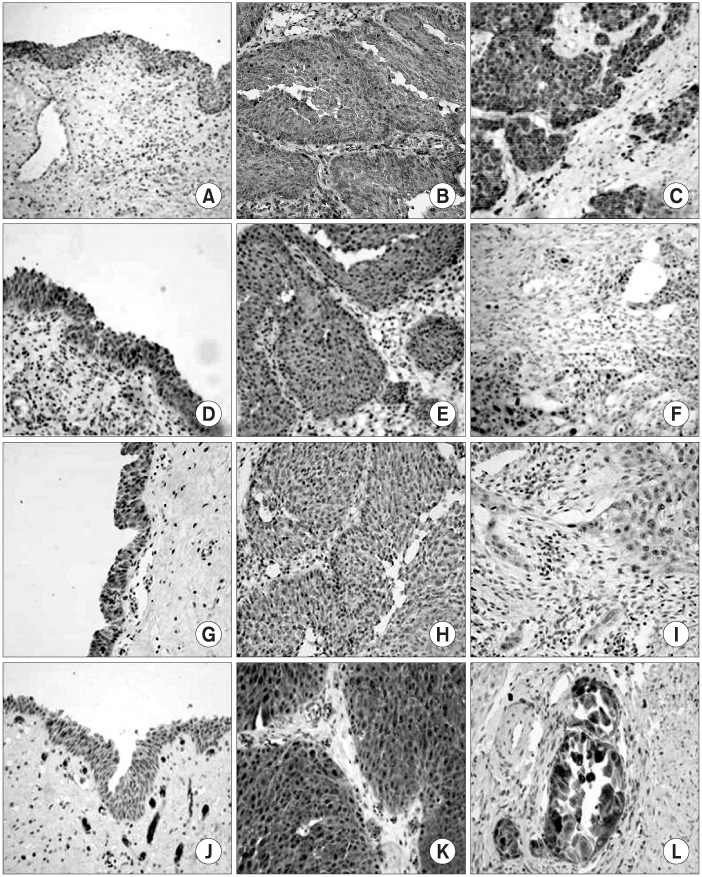
The expressions of NF-kappaB, BCL2-associated X protein (BAX), BCL2-associated death protein (BAD) and BH3-interacting domain death agonist protein (BID) were investigated by performing immunohistochemical staining on 133 archival bladder tissue paraffin blocks; these blocks included 122 transitional cell carcinomas of the urinary bladder and 11 normal bladder mucosae.
RESULTS
The expression levels of NF-kappaB were significantly higher in the bladder tumors than those of the normal bladder mucosae (p=0.001). The expression levels of BAX in the superficial and low-grade (grade 1 and 2) bladder tumors were significantly enhanced more than those of the high-grade and invasive cases (p=0.042 and p=0.045, respectively), while the expression levels of BAD in the tumor tissues and low-grade tumors were significantly elevated compared with those of the normal mucosae and high grade tumor (p=0.007 and p=0.048, respectively). But the expressions of BID were not correlated with any pathologic and clinical properties.
CONCLUSIONS
The expressions of the NF-kappaB and apoptosis inducing genes such as BAX and BAD are strongly associated with the pathogenesis and clinical properties of bladder tumor. (Korean J Urol 2007;48:483-488)
Keyword
MeSH Terms
-
Apoptosis*
bcl-2-Associated X Protein
BH3 Interacting Domain Death Agonist Protein
Carcinoma, Transitional Cell
Cell Line
Humans
Mucous Membrane
NF-kappa B*
Paraffin
Transcription Factors
Urinary Bladder Neoplasms*
Urinary Bladder*
BH3 Interacting Domain Death Agonist Protein
NF-kappa B
Paraffin
Transcription Factors
bcl-2-Associated X Protein
Figure
Reference
-
1. Huber MA, Azoitei N, Baumann B, Grunert S, Sommer A, Pehamberger H, et al. NF-kappaB is essential for epithelial-mesenchymal transition and metastasis in a model of breast cancer progression. J Clin Invest. 2004. 114:569–581.2. Parker SL, Tong T, Bolden S, Wingo PA. Cancer statistics, 1996. CA Cancer J Clin. 1996. 46:5–27.3. Edward M, Messing MD. Wein AJ, Kavoussi LR, Novic AC, Partin AW, Peters CA, editors. Urothelial tumors of the bladder. Campbell-Walsh urology. 2006. 9th ed. Philadelphia: Saunders;2407–2446.4. Kim WJ, Lee HL, Lee SC, Kim YT, Kim H. Polymorphisms of N-acetyltransferase 2, glutathione S-transferase mu and theta genes as risk factors of bladder cancer in relation to asthma and tuberculosis. J Urol. 2000. 164:209–213.5. Kim WJ, Kim H, Kim CH, Lee MS, Oh BR, Lee HM, et al. GSTT1-null genotype is a protective factor against bladder cancer. Urology. 2002. 60:913–918.6. Steiner MS. Review of peptide growth factors in benign prostatic hyperplasia and urological malignancy. J Urol. 1995. 153:1085–1096.7. Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998. 16:225–260.8. Orlowski RZ, Baldwin AS Jr. NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002. 8:385–389.9. Liou AK, Clark RS, Henshall DC, Yin XM, Chen J. To die or not to die for neurons in ischemia, traumatic brain injury and epilepsy: a review on the stress-activated signaling pathways and apoptotic pathways. Prog Neurobiol. 2003. 69:103–142.10. Niquet J, Wasterlain CG. Bim, Bad, and Bax: a deadly combination in epileptic seizures. J Clin Invest. 2004. 113:960–962.11. Sinicrope FA, Ruan SB, Cleary KR, Stephens LC, Lee JJ, Levin B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995. 55:237–241.12. Kebel AS, Reiter RE. Wein AJ, Kavoussi LR, Novic AC, Partin AW, Peters CA, editors. Molecular genetics and cancer biology. Campbell-Walsh urology. 2006. 9th ed. Philadelphia: Saunders;507–552.13. Sanchez-Beato M, Sanchez-Aguilera A, Piris MA. Cell cycle deregulation in B-cell lymphomas. Blood. 2003. 101:1220–1235.14. Karin M, Lin A. NF-kappaB at the crossroads of life and death. Nat Immunol. 2002. 3:221–227.15. Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002. 2:301–310.16. Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001. 107:241–246.17. Takeshita H, Yoshizaki T, Miller WE, Sato H, Furukawa M, Pagano JS, et al. Matrix metalloproteinase 9 expression is induced by Epstein-Barr virus latent membrane protein 1 C-terminal activation regions 1 and 2. J Virol. 1999. 73:5548–5555.18. Keely PJ, Westwick JK, Whitehead IP, Der CJ, Parise LV. Cdc42 and Rac1 induce integrin-mediated cell motility and invasiveness through PI(3)K. Nature. 1997. 390:632–636.19. Huang S, DeGuzman A, Bucana CD, Fidler IJ. Nuclear factor-kappaB activity correlates with growth, angiogenesis, and metastasis of human melanoma cells in nude mice. Clin Cancer Res. 2000. 6:2573–2581.20. Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998. 281:1322–1326.21. Chittenden T, Harrington EA, O'Connor R, Flemington C, Lutz RJ, Evan GI, et al. Induction of apoptosis by the Bcl-2 homologue Bak. Nature. 1995. 374:733–736.22. Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM. Bax-independent inhibition of apoptosis by Bcl-XL. Nature. 1996. 379:554–556.23. Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000. 6:513–519.24. Srinivasula SM, Ahmad M, Fernandes-Alnemri T, Alnemri ES. Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol Cell. 1998. 1:949–957.25. Soung YH, Lee JW, Park WS, Nam SW, Lee JY, Yoo NJ, et al. BH3 domain mutation of proapoptotic genes Bad, Bmf and Bcl-G is rare in transitional cell carcinomas of the urinary bladder. Pathology. 2006. 38:33–34.26. Zinkel S, Gross A, Yang E. BCL2 family in DNA damage and cell cycle control. Cell Death Differ. 2006. 13:1351–1359.27. Korkolopoulou P, Lazaris A, Konstantinidou AE, Kavantzas N, Patsouris E, Christodoulou P, et al. Differential expression of bcl-2 family proteins in bladder carcinomas. Relationship with apoptotic rate and survival. Eur Urol. 2002. 41:274–283.28. Martin DA, Elkon KB. Mechanisms of apoptosis. Rheum Dis Clin North Am. 2004. 30:441–454.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Differential Regulation of NF-kappaB Signaling during Human Cytomegalovirus Infection
- Inhibiting NF-kappaB Activation by Triptolide Enhances TRAIL-induced Cell Death in Lung Cancer Cells
- The Role of NF-kappaB in the TNF-alpha-induced Apoptosis of Lung Cancer Cell Line
- The Proteasome Inhibitor MG132 Sensitizes Lung Cancer Cells to TRAIL-induced Apoptosis by Inhibiting NF-kappaB Activation
- The Role of Nuclear Factor Kappa B Activation in Atherosclerosis and Ischemic Cardiac Injury