Lab Med Online.
2013 Jul;3(3):138-144. 10.3343/lmo.2013.3.3.138.
Evaluation of the Performance of LABGEO PT Hepatic Test 9
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea. wkmin@amc.seoul.kr
- KMID: 2053548
- DOI: http://doi.org/10.3343/lmo.2013.3.3.138
Abstract
- BACKGROUND
The Samsung LABGEO PT Hepatic Test 9 (Samsung electronics, Korea) was developed as a point-of-care (POC) testing device. The levels of 9 analytes, namely, albumin, AST, ALT, alkaline phosphatase, gamma-glutamyl transferase, glucose, total bilirubin, direct bilirubin, and total protein, could be evaluated simultaneously by using 70 microL of whole blood, plasma, or serum samples. In this study, we assessed the performance of the Samsung LABGEO PT Hepatic Test 9.
METHODS
The precision and linearity of the test were evaluated according to the CLSI EP5-A2 and CLSI EP6-A guidelines, respectively. Correlational analyses between Samsung LABGEO PT Hepatic Test 9 and Cobas 8000 modular analyzer (Roche, Switzerland) were carried out as per the CLSI EP9-A2 guidelines. Additionally, the results between 3 different specimen types, whole blood, plasma, and serum samples obtained from the same individual were compared to evaluate the matrix effect.
RESULTS
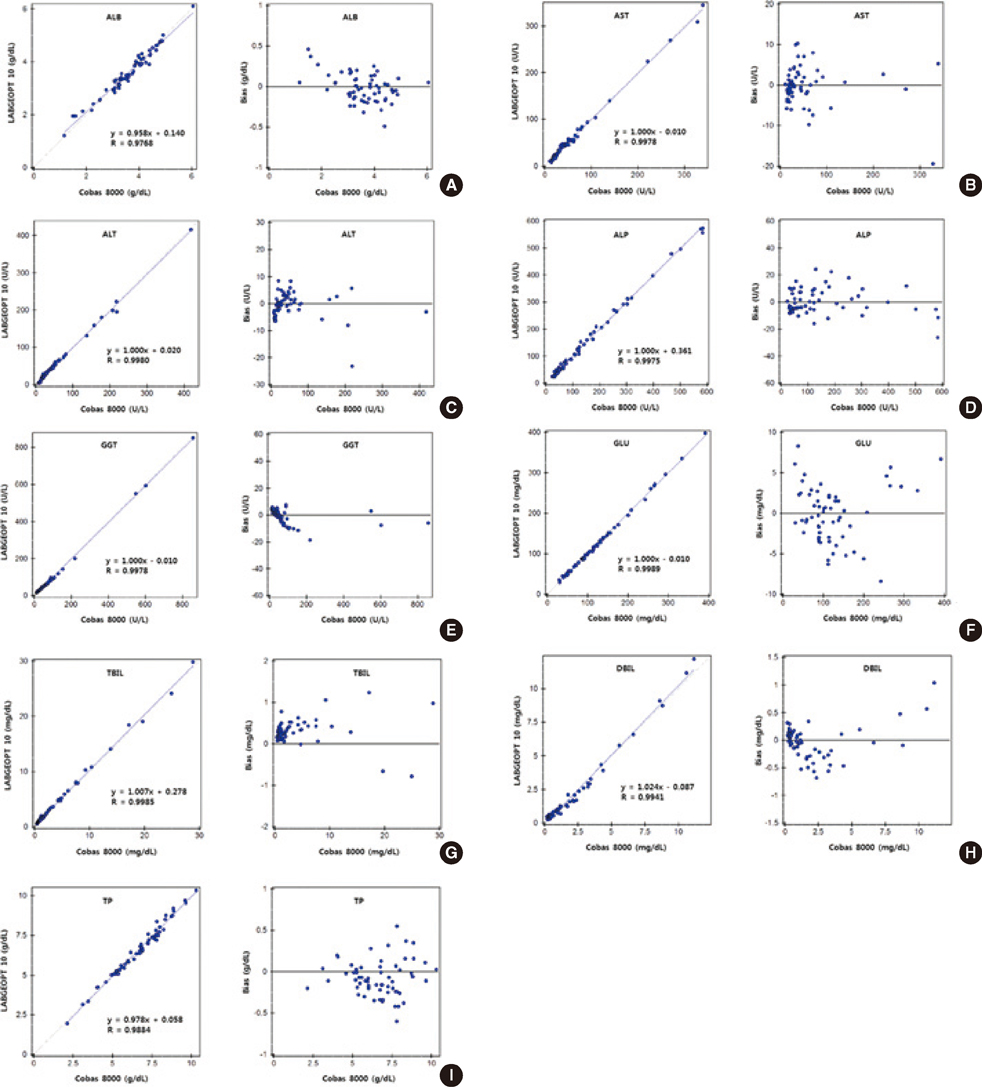
The total imprecision at both low and high levels of the 9 analytes was within 10% and in the clinically important concentration range for all test items, all obtained results were linear. We compared the above results with those obtained using Cobas 8000 and a good correlation was observed with a correlation coefficient of more than 0.975 for all 9 analytes. Simple linear regression analyses between the 3 different specimen types indicated that there was no statistically significant difference (P<0.001).
CONCLUSIONS
The Samsung LABGEO PT Hepatic Test 9 showed good precision and linearity when compared to established assays for 9 clinical test items and could be useful in cases where the POC testing is required.
MeSH Terms
Figure
Reference
-
1. Salem M, Chernow B, Burke R, Stacey JA, Slogoff M, Sood S. Bedside diagnostic blood testing. Its accuracy, rapidity, and utility in blood conservation. JAMA. 1991; 266:382–389.
Article2. Sands VM, Auerbach PS, Birnbaum J, Green M. Evaluation of a portable clinical blood analyzer in the emergency department. Acad Emerg Med. 1995; 2:172–178.
Article3. Aduen J, Bernstein WK, Khastgir T, Miller J, Kerzner R, Bhatiani A, et al. The use and clinical importance of a substrate-specific electrode for rapid determination of blood lactate concentrations. JAMA. 1994; 272:1678–1685.
Article4. Price CP, John AS, editors. Point-of-care testing making innovation work for patient-centered care. Washington DC: AACC Press;2012. p. 1–26.5. Nichols JH. Point of care testing. Clin Lab Med. 2007; 27:893–908.
Article6. Clinical and Laboratory Standards Institute. EP5-A2. Evaluation of precision performance of quantitative measurement methods; Approved guideline. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2004.7. Clinical and Laboratory Standards Institute. EP6-A. Evaluation of the linearity of quantitative measurement procedures: A statistical approach; Approved guideline. Wayne, PA: Clinical and Laboratory Standards Institute;2003.8. Clinical and Laboratory Standards Institute. EP9-A2. Method comparison and bias estimation using patient samples; Approved guideline. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2002.9. Clinical and Laboratory Standards Institute. EP14-A2. Evaluation of matrix effects; Approved guideline. 2nd ed. Wayne, PA: Clinical and Laboratory Standards Institute;2005.10. Medicare, Medicaid and CLIA programs; regulations implementing the Clinical Laboratory Improvement Amendments of 1988 (CLIA)--HCFA. Final rule with comment period. Fed Regist. 1992; 57:7002–7186.11. Xu Y, Wang L, Wang J, Liang H, Jiang X. Serum globulins contribute to the discrepancies observed between the bromocresol green and bromocresol purple assays of serum albumin concentration. Br J Biomed Sci. 2011; 68:120–125.
Article12. Duly EB, Grimason S, Grimason P, Barnes G, Trinick TR. Measurement of serum albumin by capillary zone electrophoresis, bromocresol green, bromocresol purple, and immunoassay methods. J Clin Pathol. 2003; 56:780–781.
Article13. Burtis CA, Ashwood ER, Burns DE, editors. Tietz textbook of clinical chemistry and molecular diagnotics. 5th ed. St. Louis: Elsevier Saunders;2011. p. 142–162.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Performance Evaluation of the LABGEO PT10 Point-of-care Chemistry Analyzer
- Comparing the Efficacy of Samsung LABGEO PT10 and Bio-Rad Variant II Turbo for HbA1c Measurement in Three Types of Blood Samples
- Performance Evaluation of a Point-of-care Test, ‘Samsung LABGEO PA CHF Test’, for the Amino-terminal Pro-brain Natriuretic Peptide
- Formulation of a New En Score in the Proficiency Test
- Method-Based Proficiency Test Program for Assessing Quality of Sanger Sequencing-Based Molecular Tests