Electrolyte Blood Press.
2009 Dec;7(2):31-37. 10.5049/EBP.2009.7.2.31.
Dialysis Unphysiology and Sodium Balance
- Affiliations
-
- 1Department of Internal Medicine, Hanyang University College of Medicine, Seoul, Korea. kimgh@hanyang.ac.kr
- KMID: 2052316
- DOI: http://doi.org/10.5049/EBP.2009.7.2.31
Abstract
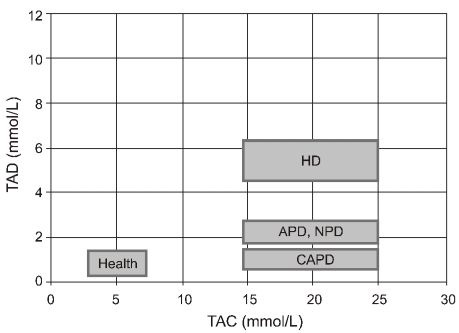
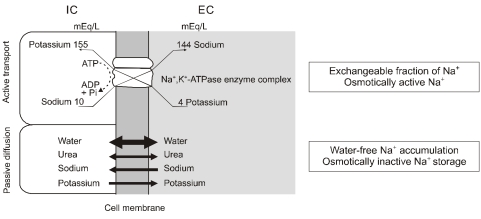
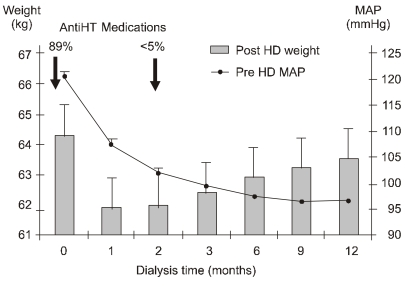
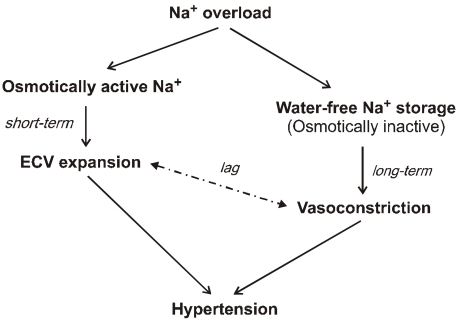
- Dialysis unphysiology was first discussed by Carl Kjellstrand in 1975 for the possible negative effects of the unphysiology of intermittent dialysis treatment. Current hemodialysis practices are still unphysiologic because they cannot keep blood chemistries within normal limits, both before and after dialysis. In addition, the discontinuous nature of hemodialysis causes saw-tooth volume fluctuations, and the extracellular fluid volume expansion during the interdialytic period may lead to hypertension and adverse cardiovascular consequences. Sodium, which is accumulated over the interdialytic period, may be divided into two fractions. The one is the fraction of osmotically active sodium which is mainly confined to the extracellular space, and the other is that of water-free (osmotically inactive) sodium which diffuses into the intracellular space. Both contribute to the pathogenesis of hypertension because the former may act to expand extracellular fluid volume and the latter may cause vasoconstriction in the long run by increasing cytosolic concentration of calcium in the vascular smooth muscle cells. Even in intensive hemodialysis, it may take several weeks to months for water-free sodium storage in the vascular smooth muscle cells to be relieved. This may be an explanation for the lag phenomenon, i.e., the delay of blood pressure decrease after normalization of extracellular fluid volume shown in the Tassin experience. Modest restriction of dietary sodium intake, the dialytic session length long enough to maintain a high ultrafiltration volume, and the reasonably low dialysate sodium concentration are required to avoid unphysiology of positive sodium balance in current hemodialysis practice.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Sodium Balance in Maintenance Hemodialysis
Seoung Woo Lee
Electrolyte Blood Press. 2012;10(1):1-6. doi: 10.5049/EBP.2012.10.1.1.
Reference
-
1. Kjellstrand CM, Evans RL, Petersen RJ, Shideman JR, von Hartitzsch B, Buselmeier TJ. The "unphysiology" of dialysis: a major cause of dialysis side effects? Kidney Int. 1975; 7(Suppl 2):30–34.
Article2. Home dialysis central [Internet]. The Medical Education Institute. c2004-2009. cited 2009 Sep 21. Available from: http://www.homedialysis.org/.3. Lopot F, Valek A. Quantification of dialysis unphysiology. Nephrol Dial Transplant. 1998; 13(Suppl 6):74–78. PMID: 9719209.
Article4. Valek A, Lopot F. Uraemic toxins and blood purification strategies. Contrib Nephrol. 1989; 70:178–187. PMID: 2766734.5. Kooman JP, van der Sande F, Leunissen K, Locatelli F. Sodium balance in hemodialysis therapy. Semin Dial. 2003; 16:351–355. PMID: 12969379.6. Charra B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007; 11:21–31. PMID: 17257351.
Article8. Stiller S, Bonnie-Schorn E, Grassmann A, Uhlenbusch-Körwer I, Mann H. A critical review of sodium profiling for hemodialysis. Semin Dial. 2001; 14:337–347. PMID: 11679103.
Article9. Edelman IS, Leibman J, O'Meara MP, Birkenfeld LW. Interrelations between serum sodium concentration, serum osmolarity and total exchangeable sodium, total exchangeable potassium and total body water. J Clin Invest. 1958; 37:1236–1256. PMID: 13575523.10. Heer M, Baisch F, Kropp J, Gerzer R, Drummer C. High dietary sodium chloride consumption may not induce body fluid retention in humans. Am J Physiol Renal Physiol. 2000; 278:F585–F595. PMID: 10751219.11. Titze J, Maillet A, Lang R, et al. Long-term sodium balance in humans in a terrestrial space station simulation study. Am J Kidney Dis. 2002; 40:508–516. PMID: 12200802.
Article12. Edelman IS, Leibman J. Anatomy of body water and electrolytes. Am J Med. 1959; 27:256–277. PMID: 13819266.
Article13. Garnett ES, Ford J, Golding PL, Mardell RJ, Whyman AE. The mobilizaton of osmotically inactive sodium during total starvation in man. Clin Sci. 1968; 35:93–103. PMID: 4878417.14. Titze J, Shakibaei M, Schafflhuber M, et al. Glycosaminoglycan polymerization may enable osmotically inactive Na+ storage in the skin. Am J Physiol Heart Circ Physiol. 2004; 287:H203–H208. PMID: 14975935.
Article15. Koomans HA, Roos JC, Boer P, Geyskes GG, Mees EJ. Salt sensitivity of blood pressure in chronic renal failure. Evidence for renal control of body fluid distribution in man. Hypertension. 1982; 4:190–197. PMID: 7040224.
Article16. Guyton AC. Kidneys and fluids in pressure regulation. Small volume but large pressure changes. Hypertension. 1992; 19(Suppl 1):I2–I8. PMID: 1730451.
Article17. Twardowski ZJ. Sodium, hypertension, and an explanation of the "lag phenomenon" in hemodialysis patients. Hemodial Int. 2008; 12:412–425. PMID: 19090863.
Article18. Murrell JR, Randall JD, Rosoff J, et al. Endogenous ouabain: upregulation of steroidogenic genes in hypertensive hypothalamus but not adrenal. Circulation. 2005; 112:1301–1308. PMID: 16116051.19. Blaustein MP, Zhang J, Chen L, Hamilton BP. How does salt retention raise blood pressure? Am J Physiol Regul Integr Comp Physiol. 2006; 290:R514–R523. PMID: 16467498.
Article20. Kempner W. Treatment of hypertensive vascular disease with rice diet. Am J Med. 1948; 4:545–577. PMID: 18909456.
Article21. Murphy RJ. The effect of "rice diet" on plasma volume and extracellular fluid space in hypertensive subjects. J Clin Invest. 1950; 29:912–917. PMID: 15436859.22. Conway J, Lauwers P. Hemodynamic and hypotensive effects of long-term therapy with chlorothiazide. Circulation. 1960; 21:21–27. PMID: 13811661.
Article23. Blumberg A, Nelp WB, Hegstrom RM, Scribner BH. Extracellular volume in patients with chronic renal disease treated for hypertension by sodium restriction. Lancet. 1967; 2:69–73. PMID: 4165465.
Article24. Charra B, Bergstrom J, Scribner BH. Blood pressure control in dialysis patients: importance of the lag phenomenon. Am J Kidney Dis. 1998; 32:720–724. PMID: 9820439.
Article25. Charra B. Fluid balance, dry weight, and blood pressure in dialysis. Hemodial Int. 2007; 11:21–31. PMID: 17257351.
Article26. Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994; 330:1431–1438. PMID: 8159199.
Article27. Khosla UM, Johnson RJ. Hypertension in the hemodialysis patient and the "lag phenomenon": insights into pathophysiology and clinical management. Am J Kidney Dis. 2004; 43:739–751. PMID: 15042553.
Article28. Perez-Garcia R, Lopez-Gomez JM, Jofre R, Junco E, Valderrabano F. Haemodialysis dose, extracellular volume control and arterial hypertension. Nephrol Dial Transplant. 2001; 16(Suppl 1):98–101. PMID: 11369833.29. Charra B, Chazot C. The neglect of sodium restriction in dialysis patients: a short review. Hemodial Int. 2003; 7:342–347. PMID: 19379386.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Sodium Balance in Maintenance Hemodialysis
- Water and Sodium Balance of Body Fluid
- Diagnosis and treatment of sodium balance disorders
- Effect of Individual Low Sodium Dialysate on Blood Pressure, Interdialytic Weight Gain, Thirst and Intradialytic Discomfort In End-Stage Renal Disease Patients
- The Effect of Cool Dialysate & Sodium Profiling on Hemodynamics in Patients with Intradialytic Hypotension