Electrolyte Blood Press.
2006 Mar;4(1):23-34. 10.5049/EBP.2006.4.1.23.
Dysregulation of ENaC in Animal Models of Nephrotic Syndrome and Liver Cirrhosis
- Affiliations
-
- 1Department of Internal Medicine, Chonnam National University Medical School, Gwangju, Korea. skimw@chonnam.ac.kr
- KMID: 2052266
- DOI: http://doi.org/10.5049/EBP.2006.4.1.23
Abstract
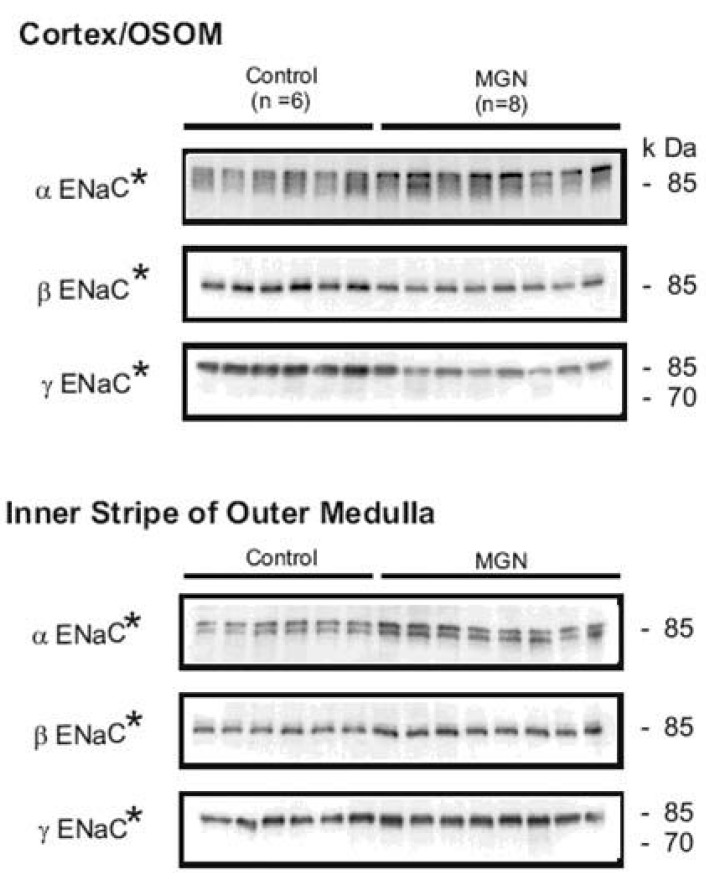
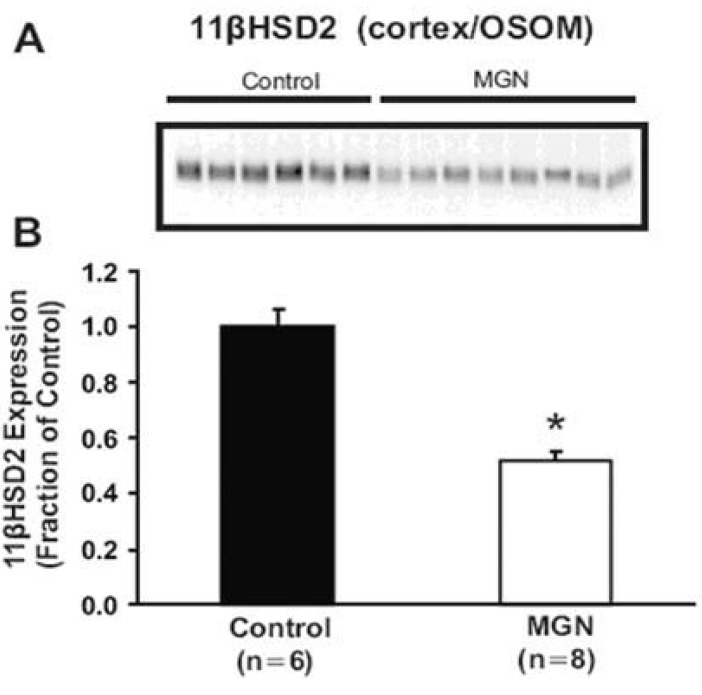
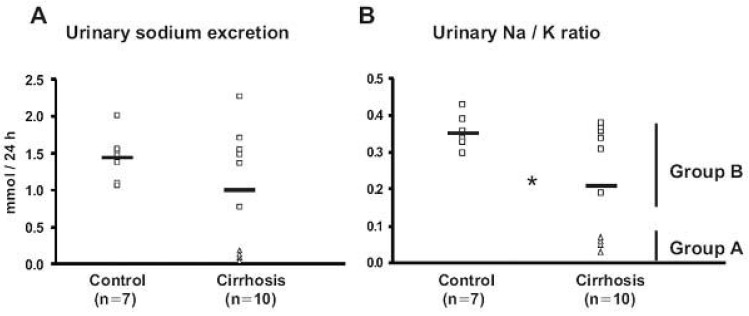
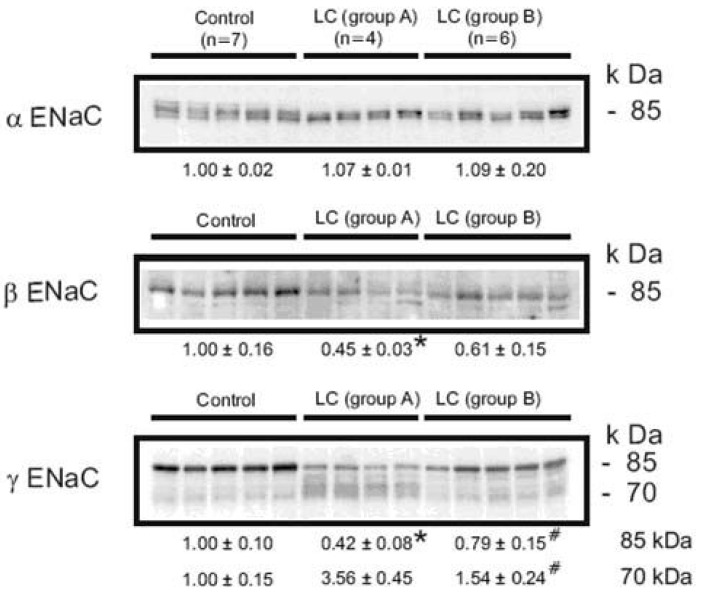
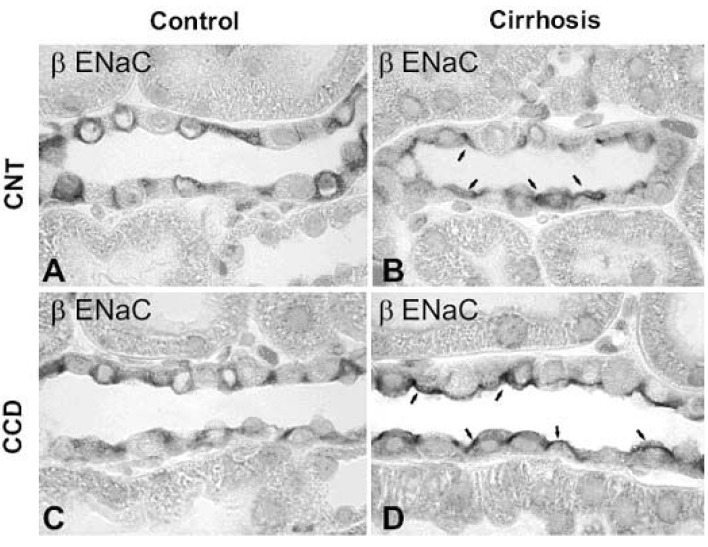
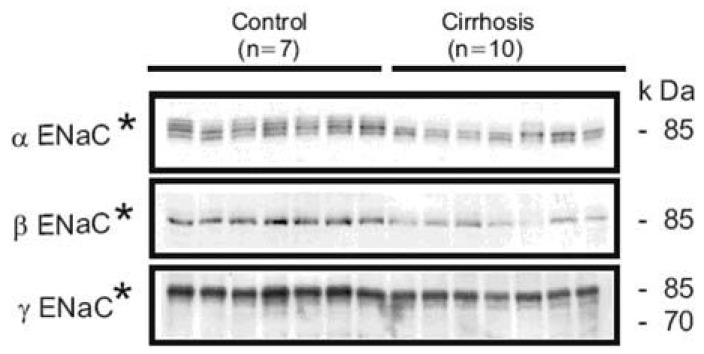
- Nephrotic syndrome and liver cirrhosis are common clinical manifestations, and are associated with avid sodium retention leading to the development of edema and ascites. However, the mechanism for the sodium retention is still incompletely understood and the molecular basis remains undefined. We examined the changes of sodium (co)transporters and epithelial sodium channels (ENaCs) in the kidneys of experimental nephrotic syndrome and liver cirrhosis. The results demonstrated that puromycin- or HgCl2?induced nephrotic syndrome was associated with 1) sodium retention, decreased urinary sodium excretion, development of ascites, and increased plasma aldosterone level; 2) increased apical targeting of ENaC subunits in connecting tubule and collecting duct segments; and 3) decreased protein abundance of type 2 11beta-hydroxysteroid dehydrogenase (11betaHSD2). Experimental liver cirrhosis was induced in rats by CCl4 treatment or common bile duct ligation. An increased apical targeting of alpha-, beta-, and gamma-ENaC subunits in connecting tubule, and cortical and medullary collecting duct segments in sodium retaining phase of liver cirhosis but not in escape phase of sodium retention. Immunolabeling intensity of 11betaHSD2 in the connecting tubule and cortical collecting duct was significantly reduced in sodium retaining phase of liver cirrhosis, and this was confirmed by immunoblotting. These observations therefore strongly support the view that the renal sodium retention associated with nephrotic syndrome and liver cirrhosis is caused by increased sodium reabsorption in the aldosterone sensitive distal nephron including the connecting tubule and collecting duct, and increased apical targeting of ENaC subunits plays a role in the development of sodium retention in nephrotic syndrome and liver cirrhosis.
MeSH Terms
-
11-beta-Hydroxysteroid Dehydrogenases
Aldosterone
Animals*
Ascites
Common Bile Duct
Edema
Epithelial Sodium Channels
Immunoblotting
Kidney
Ligation
Liver Cirrhosis*
Liver Cirrhosis, Experimental
Liver*
Models, Animal*
Nephrons
Nephrotic Syndrome*
Plasma
Rats
Sodium
United Nations
11-beta-Hydroxysteroid Dehydrogenases
Aldosterone
Epithelial Sodium Channels
Sodium
Figure
Reference
-
1. Hager H, Kwon TH, Vinnikova AK, Masilamani S, Brooks HL, Frokiaer J, Knepper MA, Nielsen S. Immunocytochemical and immunoelectron microscopic localization of alpha-, beta-, and gamma-ENaC in rat kidney. Am J Physiol. 2001; 280:F1093–F1106.2. Kim SW, Wang W, Kwon TH, Knepper MA, Frokiaer J, Nielsen S. Increased expression of ENaC subunits and increased apical targeting of AQP2 in the kidneys of spontaneously hypertensive rats. Am J Physiol Renal Physiol. 2005; 289:F957–F968. PMID: 15956775.
Article3. Kim SW, Gresz Veronika, Rojek Alexandra, Wang Weidong, Verkman A.S., Frøkiær Jørgen, Nielsen Søren. Decreased expression of AQP2 and AQP4 water channels and Na,K-ATPase in kidney collecting duct in AQP3 null mice. Biol Cell. 2005; 97:765–778. PMID: 15898956.4. Awayda MS, Tousson A, Benos DJ. Regulation of a cloned epithelial Na+ channel by its β- and γ-subunits. Am J Physiol. 1997; 273:C1889–C1899. PMID: 9435494.5. Barker PM, Nguyen MS, Gatzy JT, Grubb B, Norman H, Hummler E, Rossier B, Boucher RC, Koller B. Role of gammaENaC subunit in lung liquid clearance and electrolyte balance in newborn mice. Insights into perinatal adaptation and pseudohypoaldosteronism. J Clin Invest. 1998; 102:1634–1640. PMID: 9788978.
Article6. Ecelbarger CA, Kim GH, Terris J, Masilamani S, Mitchell C, Reyes I, Verbalis JG, Knepper MA. Vasopressin-mediated regulation of epithelial sodium channel abundance in rat kidney. Am J Physiol. 2000; 279:F46–F53.
Article7. Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA. Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest. 1999; 104:R19–R23. PMID: 10510339.8. Loffing J, Zecevic M, Feraille E, Kaissling B, Asher C, Rossier BC, Firestone GL, Pearce D, Verrey F. Aldosterone induces rapid apical translocation of ENaC in early portion of renal collecting system : possible role of SGK. Am J Physiol Renal Physiol. 2001; 280:F675–F682. PMID: 11249859.9. Duc C, Farman N, Canessa CM, Bonvalet JP, Rossier BC. Cell-specific expression of epithelial sodium channel α, β, and γ subunits in aldosterone-responsive epithelia from the rat : localization by in situ hybridization and immunocytochemistry. J Cell Biol. 1994; 127:1907–1921. PMID: 7806569.10. Garty H, Palmer LG. Epithelial sodium channels : function, structure, and regulation. Physiol Rev. 1997; 77:359–396. PMID: 9114818.11. Ichikawa I, Rennke HG, Hoyer JR, Badr KF, Schow N, Troy JL, Lechene CP, Brenner BM. Role for intrarenal mechanisms in the impaired salt excretion of experimental nephritic syndrome. J Clin Invest. 1983; 71:91–103. PMID: 6848563.12. Deschenes G, Doucet A. Collecting duct Na,K-ATPase activity correlates with urinary sodium excretion in rat nephrotic syndrome. J Am Soc Nephrol. 2000; 11:604–615. PMID: 10752519.13. Deschenes G, Gonin S, Zolty E, Cheval L, Rousselot M, Martin PY, Verbavatz JM, Feraille E, Doucet A. Increased synthesis and avp unresponsiveness of Na,K-ATPase in collecting duct from nephrotic rats. J Am Soc Nephrol. 2001; 12:2241–2252. PMID: 11675400.14. Kim SW, Wang W, Nielsen J, Praetorius J, Kwon TH, Knepper MA, Frokiaer J, Nielsen S. Increased expression and apical targeting of renal ENaC subunits in puromycin aminonucleoside-induced nephrotic syndrome in rats. Am J Physiol Renal Physiol. 2004; 286:F922–F935. PMID: 15075188.
Article15. Deschenes G, Wittner M, Stefano A, Jounier S, Doucet A. Collecting duct is a site of sodium retention in PAN nephrosis : a rationale for amiloride therapy. J Am Soc Nephrol. 2001; 12:598–601. PMID: 11181809.16. Schena FP, Gesualdo L, Grandaliano G, Montinaro V. Progression of renal damage in human glomerulonephritides : is there sleight of hand in winning the game? Kidney Int. 1997; 52:1439–1457. PMID: 9407490.17. Druet P, Druet E, Potdevin F, Sapin C. Immune type glomerulonephritis induced by HgCl2 in the Brown Norway rat. Ann Immunol. 1978; 129:777–792.18. Kim SW, de Seigneux S, Sassen M, Lee JU, Kim J, Knepper MA, Frokiaer J, Nielsen S. Increased apical targeting of renal ENaC subunits and decreased expression of 11βHSD2 in HgCl2-induced nephritic syndrome in rats. Am J Physiol Renal Physiol. (in press).19. Kalant N, Das GD, Despointes R, Giroud CJ. Mechanisms of edema in experimental nephrosis. Am J Physiol. 1962; 202:91–96. PMID: 14453227.
Article20. Pedraza-Chaverri J, Cruz C, Chavez MT, Ibarra-Rubio ME, Tapia E, Pena JC. Pathophysiology of experimental nephrotic syndrome induced by puromicyn aminonucleoside in rats. III. Effect of captopril, an angiotensin converting enzyme inhibitor, on proteinuria and sodium retention. Rev Invest Clin. 1990; 42:210–216. PMID: 2270368.21. Funder JW, Pearce PT, Smith R, Smith AI. Mineralocorticoid action: target tissue specificity is enzyme, not receptor, mediated. Science. 1988; 242:583–585. PMID: 2845584.
Article22. Vogt B, Dick B, N'Gankam V, Frey FJ, Frey BM. Reduced 11beta-hydroxysteroid dehydrogenase activity in patients with the nephrotic syndrome. J Clin Endocrinol Metab. 1999; 84:811–814. PMID: 10022459.23. Vogt B, Dick B, Marti HP, Frey FJ, Frey BM. Reduced 11beta-hydroxysteroid dehydrogenase activity in experimental nephrotic syndrome. Nephrol Dial Transplant. 2002; 17:753–758. PMID: 11981059.
Article24. Peti-peterdi J, Warnock DG, Bell PD. Angiotensin II directly stimulates ENaC activity in the cortical collecting duct via AT(1) receptors. J Am Soc Nephrol. 2002; 13:1131–1135. PMID: 11960999.25. Ghosh S, Sica D, Schoolwerth AC, Quigg RJ, Haas M, Fakhry I, Gehr TW. The role of the renin-angiotensin system in cholesterol and puromycin mediated renal injury. Am J Med Sci. 2002; 324:296–304. PMID: 12495295.
Article26. Pyo HJ, Summer SN, Niederberger M, Kim JK, Schrier RW. Arginine vasopressin gene expression in rats with puromycin-induced nephrotic syndrome. Am J Kidney Dis. 1995; 25:58–62. PMID: 7810534.
Article27. De Seigneux S, Kim SW, Sassen MC, Frøkiær J, Nielsen S. The cyclooxygenase 2 and renin-angiotensin system and sodium retention in puromycin induced nephrotic syndrome. Am J Physiol Renal Physiol. (submitted).28. Das GD, Kalant N, Giroud CJ. Experimental aminonucleoside nephrosis. II. Effect of adrenalectomy on fluid retention of aminonucleoside nephrosis. Proc Soc Exp Biol Med. 1959; 100:602–604. PMID: 13634223.29. Vogt B, Favre H. Na+,K(+)-ATPase activity and hormones in single nephron segments from nephrotic rats. Clin Sci. 1991; 80:599–604. PMID: 1647923.
Article30. Nielsen J, Kwon TH, Masilamani S, Beutler K, Hager H, Nielsen S, Knepper MA. Sodium transporter abundance profiling in kidney : effect of spironolactone. Am J Physiol Renal Physiol. 2002; 283:F923–F933. PMID: 12372767.
Article31. Gaeggeler HP, Gonzalez-Rodriguez E, Jaeger NF, Loffing-Cueni D, Norregaard R, Loffing J, Horisberger JD, Rossier BC. Mineralocorticoid versus glucocorticoid receptor occupancy mediating aldosterone-stimulated sodium transport in a novel renal cell line. J Am Soc Nephrol. 2005; 16:878–891. PMID: 15743993.32. De Seigneux S, Kim SW, Hemmingsen SC, Frøkiær J, Nielsen S. Increased expression but not targeting of ENaC in adrenalectomized rats with PAN-induced nephrotic syndrome. Am J Physiol Renal Physiol. 2006; (in press).
Article33. Wilkinson SP, Jowett TP, Slater JD, Arroyo V, Moodie H, Williams R. Renal sodium retention in cirrhosis : relation to aldosterone and nephron site. Clin Sci. 1979; 56:169–177. PMID: 477199.34. Perez-Ayuso RM, Arroyo V, Planas R, Gaya J, Bory F, Rimola A, Rivera F, Rodes J. Randomized comparative study of efficacy of furosemide versus spironolactone in nonazotemic cirrhosis with ascites. Relationship between the diuretic response and the activity of the renin-aldosterone system. Gastroenterology. 1983; 84:961–968. PMID: 6339312.35. Ecelbarger CA. Role of aldosterone-sensitive distal nephron in the sodium retention associated with liver cirrhosis. Kidney Int. 2006; 69:10–12. PMID: 16374415.36. Kim SW, Schou UK, Peters CD, de Seigneux S, Kwon TH, Knepper MA, Jonassen T, Frokiaer J, Nielsen S. Increased apical targeting of renal ENaC subunits and decreased expression of 11βHSD2 in rats with CCl4-induced decompensated liver cirrhosis. J Am Soc Nephrol. 2005; 16:3196–3210. PMID: 16192424.37. Chaimovitz C, Szylman P, Alroy G, Better OS. Mechanism of increased renal tubular sodium reabsorption in cirrhosis. Am J Med. 1972; 52:198–202. PMID: 5058505.
Article38. Kountouras J, Billing BH, Scheuer PJ. Prolonged bile duct obstruction : a new experimental model for cirrhosis in the rat. Br J Exp Pathol. 1984; 65:305–311. PMID: 6743531.39. Kim SW, Wang W, Sassen M, Choi KC, Han JS, Knepper MA, Jonassen T, Frokiaer J, Nielsen S. Biphasic changes of ENaC abundance and trafficking in CBDL induced liver cirrhosis. Kidney Int. 2006; 69:89–98. PMID: 16374428.40. Card WI, Mitchell W, Strong JA, Taylor NR, Tompsett SL, Wilson JM. Effects of liquorice and its derivatives on salt and water metabolism. Lancet. 1953; 1:663–668. PMID: 13036120.
Article41. Turpie AG, Thomson TJ. Carbenoxolone sodium in the treatment of gastric ulcer with special reference to side-effects. Gut. 1965; 6:591–594. PMID: 5323391.
Article42. Quattropani C, Vogt B, Odermatt A, Dick B, Frey BM, Frey FJ. Reduced activity of 11 beta-hydroxysteroid dehydrogenase in patients with cholestasis. J Clin Invest. 2001; 108:1299–1305. PMID: 11696574.43. Escher G, Nawrocki A, Staub T, Vishwanath BS, Frey BM, Reichen J, Frey FJ. Down-regulation of hepatic and renal 11 beta-hydroxysteroid dehydrogenase in rats with liver cirrhosis. Gastroenterology. 1998; 114:175–184. PMID: 9428231.44. Kim SW, Hemingsen SC, de Seigneux S, Sassen MC, Kwon TH, Knepper MA, Frokiaer J, Nielsen S. Increased apical targeting of ENaC subunits in rats with inhibited 11βHSD2 activity by glycyrrhizic acid. Am J Physiol Renal Physiol. (submitted).45. Hellal-Levy C, Fagart J, Souque A, Rafestin-Oblin ME. Mechanistic aspects of mineralocorticoid receptor activation. Kidney Int. 2000; 57:1250–1255. PMID: 10760050.
Article46. Lombes M, Kenouch S, Souque A, Farman N, Rafestin-Oblin ME. The mineralocorticoid receptor discriminates aldosterone from glucocorticoids independently of the 11 beta-hydroxysteroid dehydrogenase. Endocrinology. 1994; 135:834–840. PMID: 8070376.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A case of rhinocerebral mucormycosis associated with liver cirrhosis and nephrotic syndrome
- Pathophysiological Implications of Sodium Transporters and Water Channels in the Kidney
- A Case of Crescentic IgA Nephropathy Associated with Alcoholic Liver Cirrhosis
- A Study on Changes of Serum HDL-Cholesterol Level in Some Diseases
- Experimental Animal Models of Portal Hypertension