Ann Clin Microbiol.
2015 Sep;18(3):88-93. 10.5145/ACM.2015.18.3.88.
Activities of Quality Improvement for Blood Culture at a University Hospital
- Affiliations
-
- 1Department of Laboratory Medicine, Soonchunhyang University Hospital, Seoul, Korea. choity@schmc.ac.kr
- 2Division of Infectious Diseases, Department of Internal Medicine, Soonchunhyang University Hospital, Seoul, Korea.
- KMID: 2052102
- DOI: http://doi.org/10.5145/ACM.2015.18.3.88
Abstract
- BACKGROUND
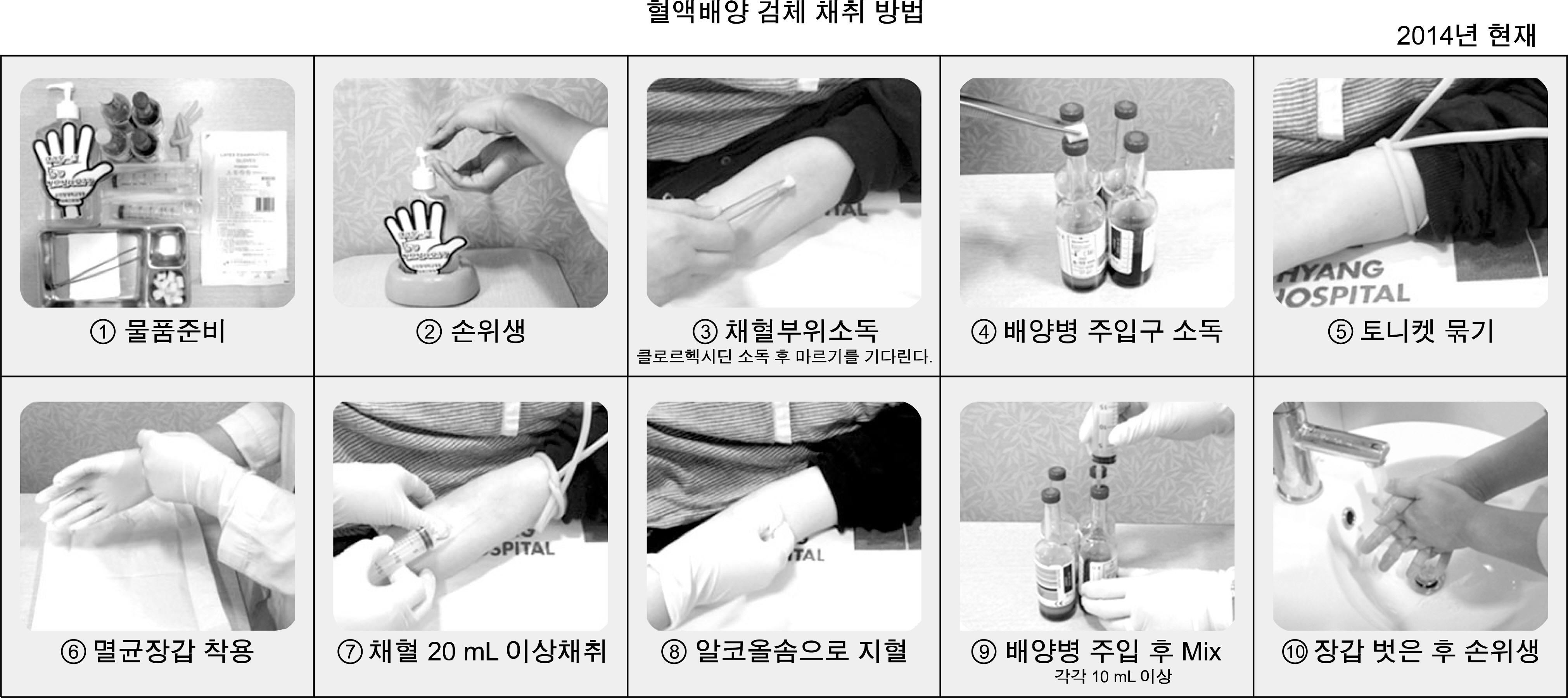
Blood culture is a critical test for diagnosing bloodstream infections. Frequent microbial contamination during sampling and testing leads to abuse of antimicrobial agents. We evaluated methods for reducing contamination and obtaining more reliable results.
METHODS
We analyzed blood cultures obtained between 2009 and 2015. We established 6 quality indicators: true positive rate, contamination rate, blood sampling volume, number of sets of blood cultures, delayed transportation rate, and percentage of samples collected from the femoral region, with reference to the CLSI guideline M47-A, 2007. Education was provided for interns and nurses responsible for blood sampling and transportation of specimens, and data were analyzed monthly.
RESULTS
At baseline, the true positive rate was 12.8%, and the contamination rate was 4.0%. During the intervention period, these were decreased to 10.9% and 1.9%, respectively. The percentage of samples smaller than 5 mL decreased from 29.7% to 2.7-11.3%. The rate of one set of blood cultures being ordered was always <5%. The delayed transportation rate decreased from 35.6% to 5.5-7.7%. Finally, the percentage of samples collected from the femoral region decreased from 41.5% to 22.0-31.0%, because of which we did not attain our goal, 20.8%.
CONCLUSION
The results showed improvements in contamination rate, specimen volume, specimen transportation time, and the percentage of samples collected from the femoral region. The quality management of blood cultures in 2011 was comparatively poor, which led to increased contamination rate, large number of samples containing <5 mL of blood, and increased percentage of samples collected from the femoral region. Thus, quality improvement methods can produce more reliable results of blood cultures.
MeSH Terms
Figure
Cited by 1 articles
-
Effects of Blood Volume Monitoring on the Rate of Positive Blood Cultures from the Emergency Room
Min-Kyung So, Hee Jung Choi, Miae Lee, Min-Kyung So, Hae-Sun Chung, Chung-Jong Kim, Hee Jung Choi, Miae Lee
Ann Clin Microbiol. 2016;19(3):70-76. doi: 10.5145/ACM.2021.19.3.70.
Reference
-
1.Hall KK., Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006. 19:788–802.
Article2.CLSI. Principles and procedures for blood cultures; approved guideline. CLSI document M47-A. Wayne, PA: Clinical and Laboratory Standards Institute. 2007.3.Mimoz O., Karim A., Mercat A., Cosseron M., Falissard B., Parker F, et al. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture. A randomized, controlled trial. Ann Intern Med. 1999. 131:834–7.4.Kim S. Development of blood culture and quality improvement. Ann Clin Microbiol. 2013. 16:153–61.
Article5.Garner JS., Jarvis WR., Emori TG., Horan TC., Hughes JM. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988. 16:128–40.
Article6.Weinstein MP., Towns ML., Quartey SM., Mirrett S., Reimer LG., Parmigiani G, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997. 24:584–602.
Article7.Cho YK., Kim DS., Choi SI., Lee HS. The influence of antimicrobial abuse to blood culture. Korean J Clin Microbiol. 1998. 1:63–7.8.Towns ML., Jarvis WR., Hsueh PR. Guidelines on blood cultures. J Microbiol Immunol Infect. 2010. 43:347–9.
Article9.Shin JH., Song SA., Kim MN., Kim S. Nationwide survey of blood culture performance regarding skin disinfection, blood collection and laboratory procedures. Korean J Clin Microbiol. 2011. 14:91–6.
Article10.Riedel S., Carroll KC. Blood cultures: key elements for best practices and future directions. J Infect Chemother. 2010. 16:301–16.
Article11.Li J., Plorde JJ., Carlson LG. Effects of volume and periodicity on blood cultures. J Clin Microbiol. 1994. 32:2829–31.
Article12.Cockerill FR 3rd., Wilson JW., Vetter EA., Goodman KM., Tor-gerson CA., Harmsen WS, et al. Optimal testing parameters for blood cultures. Clin Infect Dis. 2004. 38:1724–30.
Article13.Weinstein MP., Reller LB., Murphy JR., Lichtenstein KA. The clinical significance of positive blood cultures: a comprehensive analysis of 500 episodes of bacteremia and fungemia in adults. I. Laboratory and epidemiologic observations. Rev Infect Dis. 1983. 5:35–53.
Article14.Washington JA 2nd. Blood cultures: principles and techniques. Mayo Clin Proc. 1975. 50:91–8.15.Darouiche RO., Wall MJ Jr., Itani KM., Otterson MF., Webb AL., Carrick MM, et al. Chlorhexidine-alcohol versus povidone-iodine$$$$.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Development of Blood Culture and Quality Improvement
- Collaborative Quality Improvement in Neo natal Intensive Care Units
- Effects of Nurses' Patient Safety Management Importance, Patient Safety Culture and Nursing Service Quality on Patient Safety Management Activities in Tertiary Hospitals
- Comparison of Blood Culture Contamination Rate Before and After Improving Skin Antisepsis Methods in Blood Culture
- A review of the relationship between patient safety culture and safety activities: A systematic review focusing on the Korean version of the Hospital Survey on Patient Safety Culture 1.0