Chonnam Med J.
2011 Aug;47(2):80-84. 10.4068/cmj.2011.47.2.80.
Prospective Randomization Trial of G-CSF-Primed Induction Regimen versus Standard Regimen in Patients with AML
- Affiliations
-
- 1Department of Hematology/Oncology and Stem Cell Transplantation Unit, Kyungpook National University Hospital, Kyungpook National University School of Medicine, Daegu, Korea. sksohn@knu.ac.kr
- KMID: 2048789
- DOI: http://doi.org/10.4068/cmj.2011.47.2.80
Abstract
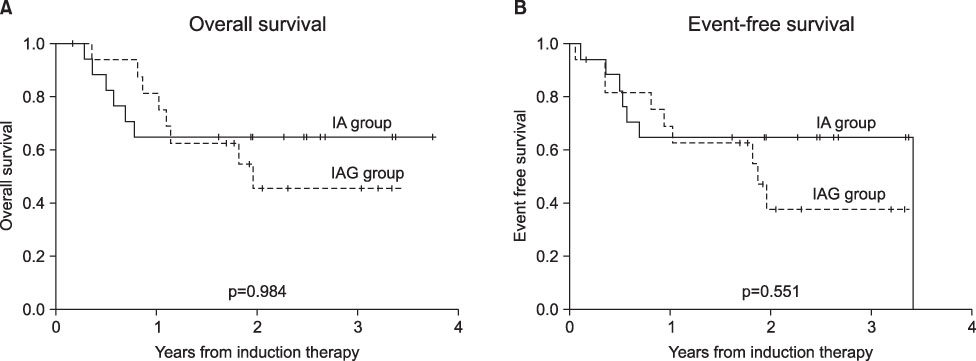
- The sensitization of leukemia cells with hematopoietic growth factors can enhance the cytotoxicity of chemotherapy in acute myeloid leukemia (AML). Therefore, the current trial attempted to evaluate the efficacy of granulocyte colony-stimulating factor (G-CSF) priming in remission induction chemotherapy with an intensified dose of Ara-C for newly diagnosed AML. Patients with newly diagnosed AML were randomly assigned to receive idarubicin (12 mg/m2/24 hr, days 1-3) plus Ara-C (500 mg/m2/12 hr, days 4-8) with G-CSF (250 microg/m2/d, days 3-7) (IAG group) or standard idarubicin (12 mg/m2/24 hr, days 1-3) plus Ara-C (100 mg/m2/12 hr, days 1-7) without G-CSF (IA group). There were no significant differences in sex, age, subtype, or cytogenetic risk between the two groups. Complete remission was achieved in 15 patients (88.2%) from the IAG group and in 14 patients (82.4%) from the IA group (p=0.31). The median time to complete remission was 26 vs. 31 days (p=0.779) for the IA and IAG groups, respectively. The median time to neutrophil recovery (>1x10(9)/L) and platelet recovery (>20x10(9)/L) did not differ significantly between the two groups (26 vs. 26 days, p=0.338; 21 vs. 16 days, p=0.190, respectively). After a median follow-up of 682 days, the 3-year overall survival rate for the IA group was 64.7%, whereas that for the IAG group was 45.6% (p=0.984). No improved clinical outcomes were observed for the AML patients subjected to intensified remission induction with G-CSF priming when compared with standard induction chemotherapy.
Keyword
MeSH Terms
-
Blood Platelets
Cytarabine
Cytogenetics
Follow-Up Studies
Granulocyte Colony-Stimulating Factor
Humans
Idarubicin
Induction Chemotherapy
Intercellular Signaling Peptides and Proteins
Leukemia
Leukemia, Myeloid, Acute
Neutrophils
Prospective Studies
Random Allocation
Remission Induction
Survival Rate
Cytarabine
Granulocyte Colony-Stimulating Factor
Idarubicin
Intercellular Signaling Peptides and Proteins
Figure
Reference
-
1. Preisler HD, Rustum Y, Henderson ES, Bjornsson S, Creaven PJ, Higby DJ, et al. Treatment of acute nonlymphocytic leukemia: use of anthracycline-cytosine arabinoside induction therapy and comparison of two maintenance regimens. Blood. 1979. 53:455–464.
Article2. Keating MJ, Smith TL, McCredie KB, Bodey GP, Hersh EM, Gutterman JU, et al. A four-year experience with anthracycline, cytosine arabinoside, vincristine and prednisone combination chemotherapy in 325 adults with acute leukemia. Cancer. 1981. 47:2779–2788.
Article3. Rai KR, Holland JF, Glidewell OJ, Weinberg V, Brunner K, Obrecht JP, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981. 58:1203–1212.
Article4. Ohno R, Naoe T, Kanamaru A, Yoshida M, Hiraoka A, Kobayashi T, et al. Kohseisho Leukemia Study Group. A double-blind controlled study of granulocyte colony-stimulating factor started two days before induction chemotherapy in refractory acute myeloid leukemia. Blood. 1994. 83:2086–2092.
Article5. Zittoun R, Suciu S, Mandelli F, de Witte T, Thaler J, Stryckmans P, et al. Granulocyte-macrophage colony-stimulating factor associated with induction treatment of acute myelogenous leukemia: a randomized trial by the European Organization for Research and Treatment of Cancer Leukemia Cooperative Group. J Clin Oncol. 1996. 14:2150–2159.
Article6. Löwenberg B, Boogaerts MA, Daenen SM, Verhoef GE, Hagenbeek A, Vellenga E, et al. Value of different modalities of granulocyte-macrophage colony-stimulating factor applied during or after induction therapy of acute myeloid leukemia. J Clin Oncol. 1997. 15:3496–3506.
Article7. te Boekhorst PA, Löwenberg B, Vlastuin M, Sonneveld P. Enhanced chemosensitivity of clonogenic blasts from patients with acute myeloid leukemia by G-CSF, IL-3 or GM-CSF stimulation. Leukemia. 1993. 7:1191–1198.8. Löwenberg B, van Putten W, Theobald M, Gmür J, Verdonck L, Sonneveld P, et al. Dutch-Belgian Hemato-Oncology Cooperative Group. Swiss Group for Clinical Cancer Research. Effect of priming with granulocyte colony-stimulating factor on the outcome of chemotherapy for acute myeloid leukemia. N Engl J Med. 2003. 349:743–752.
Article9. Rowe JM, Neuberg D, Friedenberg W, Bennett JM, Paietta E, Makary AZ, et al. Eastern Cooperative Oncology. A phase 3 study of three induction regimens and of priming with GM-CSF in older adults with acute myeloid leukemia: a trial by the Eastern Cooperative Oncology Group. Blood. 2004. 103:479–485.
Article10. Baek JH, Sohn SK, Kim DH, Kim JG, Yang DH, Kim YK, et al. Pilot remission induction therapy with idarubicin, plus an intensified dose of ara-C and priming with granulocyte colony-stimulating factor for acute myeloid leukemia. Acta Haematol. 2007. 117:109–114.
Article11. Cheson BD, Bennett JM, Kopecky KJ, Büchner T, Willman CL, Estey EH, et al. International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003. 21:4642–4649.
Article12. Büchner T, Hiddemann W, Wörmann B, Löffler H, Gassmann W, Haferlach T, et al. Double induction strategy for acute myeloid leukemia: the effect of high-dose cytarabine with mitoxantrone instead of standard-dose cytarabine with daunorubicin and 6-thioguanine: a randomized trial by the German AML Cooperative Group. Blood. 1999. 93:4116–4124.13. Cahn JY, Labopin M, Sierra J, Blaise D, Reiffers J, Ferrant A, et al. No impact of high-dose cytarabine on the outcome of patients transplanted for acute myeloblastic leukaemia in first remission. Acute Leukaemia Working Party of the European Group for Blood and Marrow Transplantation (EBMT). Br J Haematol. 2000. 110:308–314.
Article14. Amadori S, Suciu S, Jehn U, Stasi R, Thomas X, Marie JP, et al. EORTC/GIMEMA Leukemia Group. Use of glycosylated recombinant human G-CSF (lenograstim) during and/or after induction chemotherapy in patients 61 years of age and older with acute myeloid leukemia: final results of AML-13, a randomized phase-3 study. Blood. 2005. 106:27–34.
Article15. Bishop JF, Matthews JP, Young GA, Szer J, Gillett A, Joshua D, et al. A randomized study of high-dose cytarabine in induction in acute myeloid leukemia. Blood. 1996. 87:1710–1717.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Trial of Short Course Chemotherapy for Pulmonary Tuberculosis
- Therapeutic Results of Two Regimens for Childhood Acute Myelogenous Leukemia
- Mitoxantrone Based Chemotherapy Regimen for Remission Induction in Children with Refractory Acute Myeloid Leukemia
- Randomization in clinical studies
- A noninferiority, randomized controlled trial of late conversion to once-daily regimen of sirolimus and extended-release tacrolimus versus mycophenolic acid and extended-release tacrolimus for kidney transplant recipients