Korean J Obstet Gynecol.
2011 Apr;54(4):218-222. 10.5468/KJOG.2011.54.4.218.
Ovarian dysgerminoma associated with pregnancy
- Affiliations
-
- 1Department of Obstetrics and Gynecology, St. Vincent's Hospital, The Catholic University of Korea School of Medicine, Suwon, Korea. dcpark@catholic.ac.kr
- 2Department of Pathology, St. Vincent's Hospital, The Catholic University of Korea School of Medicine, Suwon, Korea.
- KMID: 2013245
- DOI: http://doi.org/10.5468/KJOG.2011.54.4.218
Abstract
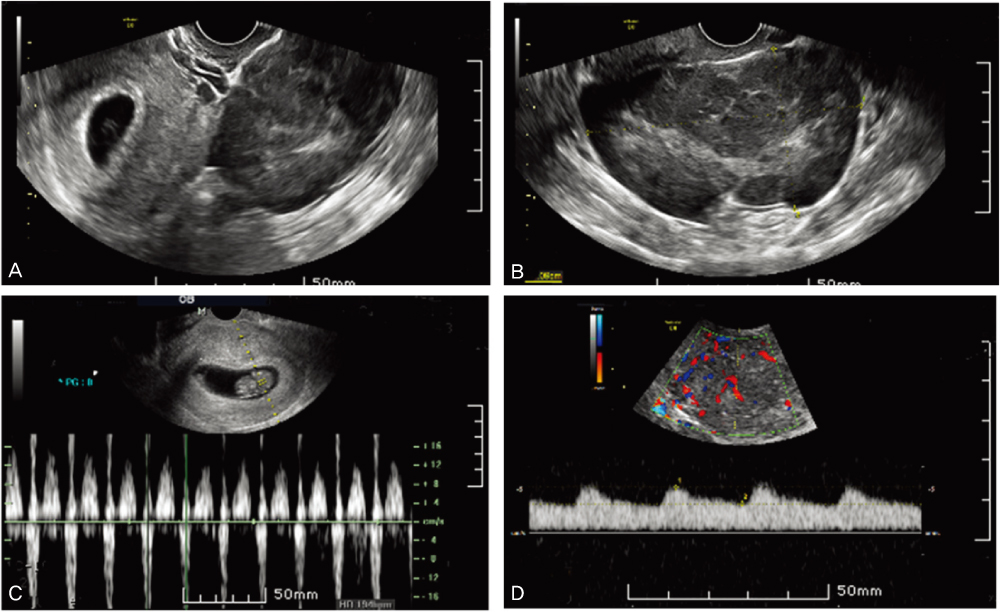
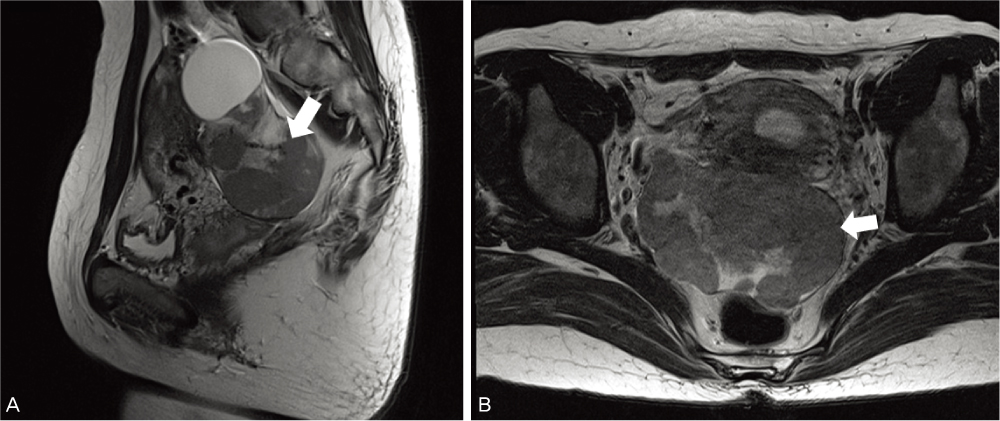
- A case of a 33-year-old 3 gravida, 1 para pregnant woman in the 7th week of gestation who was concurrented with dysgerminoma is presented. Measurements were made on serum lactic dehydrogenase, alpha-fetoprotein, CA-125, and pelvicmagnetic resonance imaging. The clinical stage was IA and right salpingo-oophorectomy and biopsy of the left ovary were done preserving the pregnancy. A cytological finding was non-specific. The frequency of malignant ovarian tumors associated with pregnancy and the treatment for it were discussed.
Keyword
MeSH Terms
Figure
Reference
-
1. Creasman WT, Rutledge F, Smith JP. Carcinoma of the ovary associated with pregnancy. Obstet Gynecol. 1971. 38:111–116.2. Beischer NA, Buttery BW, Fortune DW, Macafee CA. Growth and malignancy of ovarian tumours in pregnancy. Aust N Z J Obstet Gynaecol. 1971. 11:208–220.3. Park BW, Song SS, Lee HD. A case of a huge dysgerminoma of the ovary. Korean J Obstet Gynecol. 1983. 26:109–111.4. Kim SY, Kim YS, Park YH, Jeon K, Lim SG, Kim JW. A case of recurrent ovarian dysgerminora. Korean J Obstet Gynecol. 1983. 26:965–967.5. Kim JY, Choi SL, Park IW, Jun HC, Jung DS, Jo JD, et al. A case of pure dysgerminoma with syncytiotrophoblastic giant cell secreating hCG. Korean J Obstet Gynecol. 2003. 46:469–473.6. Bakri YN, Ezzat A, Akhtar , Dohami , Zahrani . Malignant germ cell tumors of the ovary. Pregnancy considerations. Eur J Obstet Gynecol Reprod Biol. 2000. 90:87–91.7. DiSaia PJ, Townsend DE, Morrow CP. The rationale for less than radical treatment for gynecologic malignancy in early reproductive years. Obstet Gynecol Surv. 1974. 29:581–593.8. Munnell EW. Primary ovarian cancer associated with pregnancy. Clin Obstet Gynecol. 1963. 30:983–993.9. Graber EA, Barber HR. Barber HR, Graber EA, editors. Ovarian tumors in pregnancy. Surgical disease in pregnancy. 1974. Philadelphia (PA): W.B. Saunders;428–439.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Pure Dysgerminoma with Syncytiotrophoblastic Giant Cell Secreating HCG
- Torsion and ruptured dysgerminoma of ovary in pregnancy
- Radiation Therapy of Ovarian Dysgerminoma
- Pelviscopic Diagnosis and Treatment of Two Cases of Ovarian Pregnancy
- A Case of Dysgerminoma Incidentally Found after Pelviscopic Ovarian Surgery