J Korean Diabetes Assoc.
2007 Mar;31(2):163-174. 10.4093/jkda.2007.31.2.163.
Efficacy and Safety of Mitiglinide in Korean Type 2 Diabetic Patients: Prospective Randomised Multicenter Comparative Phase III Study
- Affiliations
-
- 1Department of Internal Medicine, Eulji University School of Medicine, Korea.
- 2Department of Internal Medicine, Korea University College of Medicine, Korea.
- 3Department of Internal Medicine, The Catholic University of Korea College of Medicine, Korea.
- 4Department of Internal Medicine, Yonsei University College of Medicine, Korea.
- KMID: 2008086
- DOI: http://doi.org/10.4093/jkda.2007.31.2.163
Abstract
-
BACKGROUND: Mitiglinide, one of the meglitinides, is expected to prevent postprandial hyperglycemia of type 2 diabetes by enhancing early phase insulin secretion. The aim of this study was to verify the efficacy and safety of mitiglinide compared to nateglinide.
METHODS
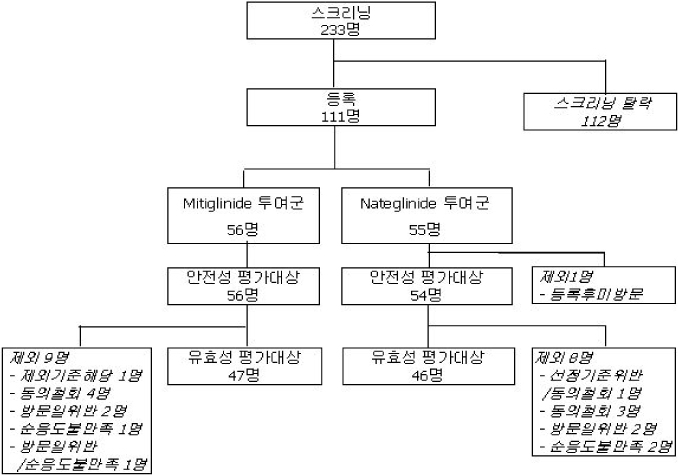
One hundred eleven of diabetic patients were randomised and administered of mitiglinide (n = 56) and nateglinide (n = 55) before a meal time for 12 weeks. The changes of HbA1c, fasting plasma glucose (FPG) and postprandial plasma glucose (PPG) were analyzed. The safety of this drug was investigated as well.
RESULTS
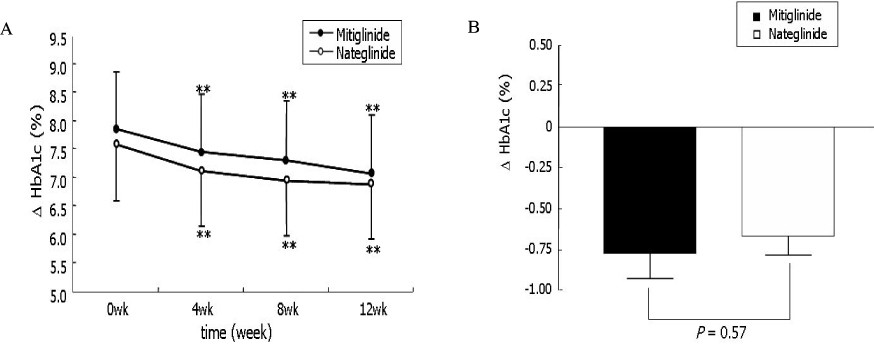
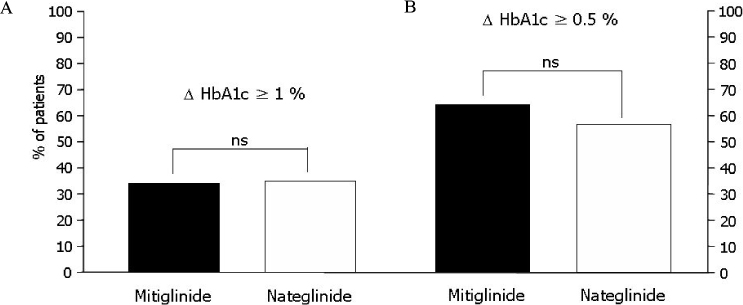
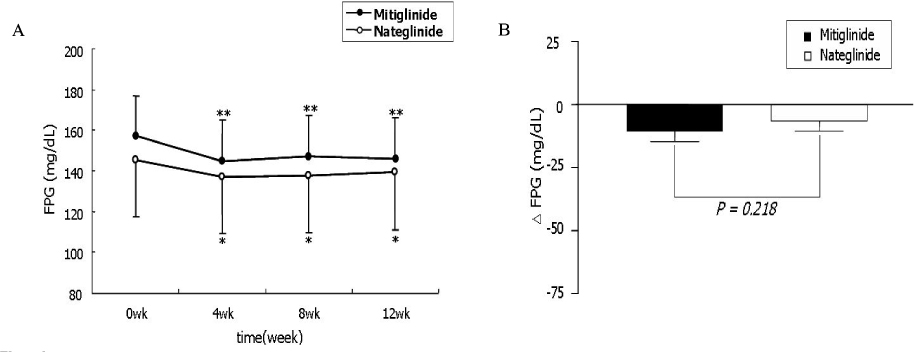
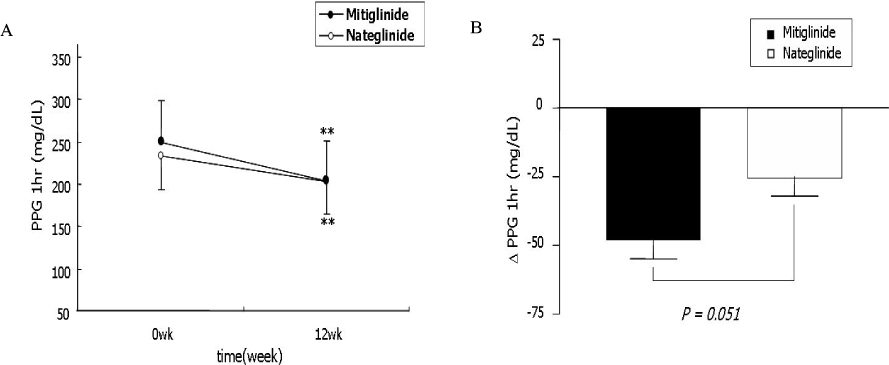
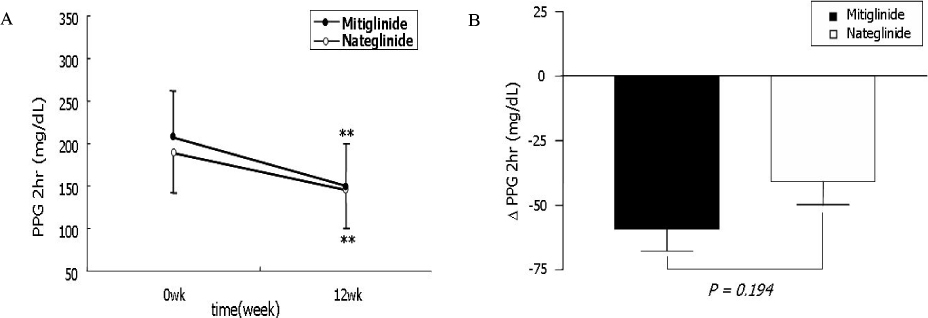
The change of HbA1c was not significantly different between two groups (-0.77 +/- 1.08% in mitiglinide vs. -0.66 +/- 0.79% in nateglinide, P = 0.57). The reduction of FPG (-12.2 +/- 25.0 mg/dL vs. -6.1 +/- 22.3 mg/dL, P = 0.218), PPG 1 hr (-48.0 +/- 47.1 mg/dL, vs. -29.4 +/- 43.2 mg/dL, P = 0.051), and PPG 2 hr (-59.2 +/- 58.0 mg/dL vs. -43.3 +/- 59.0 mg/dL, P = 0.194) were not significantly different between the mitiglinide and the nateglinide, respectively. Drug-related adverse effects were not different between two groups (16.1% in mitiglinide vs. 27.8% in nateglinide, P = 0.137). The frequency of hypoglycemic events were not different between two groups (8.9% in mitiglinide vs. 14.8% in nateglinide, P = 0.339). There were two patients who had complained shoulder pain in the mitiglinide or deterioration of visual acuity in the nateglinide, but those were found to be unrelated with medications.
CONCLUSION
This study showed that mitiglinide had reduced HbA1c as similar to nateglinide and that significantly improved HbA1c, FPG and PPG during 12 weeks of treatment. The safety of mitiglinide was also comparable to nateglinide. Mitiglinide could be used as an effective glucose-lowering agent by enhancing early insulin secretion and reducing postprandial glucose excursion, and thereby might contribute long-term cardioprotective effect in Korean type 2 diabetic patients.
Keyword
MeSH Terms
Figure
Cited by 2 articles
-
Monotherapy in Patients with Type 2 Diabetes Mellitus
Sang Youl Rhee, Hyun Jin Kim, Seung-Hyun Ko, Kyu-Yeon Hur, Nan-Hee Kim, Min Kyong Moon, Seok-O Park, Byung-Wan Lee, Kyung Mook Choi, Jin Hwa Kim,
Diabetes Metab J. 2017;41(5):349-356. doi: 10.4093/dmj.2017.41.5.349.Monotherapy in Type 2 Diabetes Mellitus Patients 2017: A Position Statement of the Korean Diabetes Association
Sang Youl Rhee
J Korean Diabetes. 2018;19(1):15-22. doi: 10.4093/jkd.2018.19.1.15.
Reference
-
2. The Diabetes Control and Complication Trial (DCCT) Research Group. Effect of intensive treatment of diabetes on the development and progression of long-term complication in insulin-dependent diabetes mellitus. N Eng J Med. 1993. 329:977–986.3. Shichiri M. Long-term results of the Fumamoto Study on optimal diabetes control in type 2 diabetic patients. Diabetes Care. 2000. 23:Suppl 2. B21–B29.4. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet. 1998. 352:837–853.5. UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998. 352:854–865.6. Balkau B, Shipley M, Jarrett RJ, Pyorala K, Pyorala M, Forhan A, Eschwege E. High blood glucose concentration is a risk factor for mortality in middle-aged nondiabetic men. 20-years follow-up in the Whitehall Study, the Paris Prospective Study, and the Helsinki Policemen Study. Diabetes Care. 1998. 21:360–367.7. The DECODE Study Group. Glucose tolerance and mortality: comparison of WHO and American diabetes association diagnostic criteria. Lancet. 1999. 354:617–621.8. Tominaga M. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose, the Funagata Diabetes Study. Diabetes Care. 1999. 22:920–924.9. Malaisse WJ. Pharmacology of the meglitinide analogs: new treatment options for type 2 diabetes mellitus. Treat Endocrinol. 2003. 2:401–414.10. Landgraf R. Meglitinide analogues in the treatment of type 2 diabetes mellitus. Drugs Aging. 2000. 17:411–425.12. Taki H, Maki T, Iso T, Iwamoto K, Kajiura T. Study of nateglinide in Japan: long-term treatment of patients with type 2 diabetes. Adv Ther. 2006. 23:307–324.13. Yalow RS, Berson SA. Plasma insulin concentrations in nondiabetic and early diabetic subjects. Determinations by a new sensitive immuno-assay technic. Diabetes. 1960. 9:254–260.14. Perley MJ, Kipnis DM. Plasma insulin responses to oral and intravenous glucose: studies in normal and diabetic sujbjects. J Clin Invest. 1967. 46:1954–1962.15. Hirose T. Significance of insulin secretion pattern lectured by "glinides" in the treatment of postprandial hyperglycemia. Nippon Rinsho. 2005. 63:2237–2244.16. Bonora E, Muggeo M. Postprandial blood glucose as a risk factor for cardiovascular disease in Type II diabetes: the epidemiological evidence. Diabetologia. 2001. 44:2107–2114.17. Avignon A, Radauceanu A, Monnier L. Nonfasting plasma glucose is a better marker of diabetic control than fasting plasma glucose in type 2 diabetes. Diabetes Care. 1997. 20:1822–1826.18. Sievers ML, Bennett PH, Nelson RG. Effect of glycemia on mortality in Pima Indians with type 2 diabetes. Diabetes. 1999. 48:896–902.19. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trail Research Group. Acarbose for prevention of type 2 diabetes mellitus: the STOP NIDDM randomised trial. Lancet. 2002. 359:2072–2077.20. Chiasson JL, Josse RG, Gomis R, Hanefeld M, Karasik A, Laakso M. STOP-NIDDM Trial Research Group. Acarbose treatment and the risk of cardiovascular disease and hypertension in patients with impaired glucose tolerance: the STOP NIDDM trial. JAMA. 2003. 290:486–494.21. Hanefeld M, Chiasson JL, Koehler C, Henkel E, Schaper F, Temelkova-Kurktschiev T. Acarbose slows progression of intima media thickness of the carotid arteries in subjects with impaired glucose tolerance. Stroke. 2004. 35:1073–1078.22. Zeymer U, Schwarzmaier-D'assie A, Petzinna D, Chiasson JL. STOP-NIDDM Trial Research Group. Effect of acarbose treatment on the risk of silent myocardial infarctions in patients with impaired glucose tolerance: results of the randomised STOP-NIDDM trial electrocardiography substudy. Eur J Cardiovasc Prev Rehabil. 2004. 11:412–415.23. Esposito K, Giugliano D, Nappo F, Marfella R. Campanian Postprandial Hyperglycemia Study Group. Regression of carotid atherosclerosis by control of postprandial hyperglycemia in type 2 diabetes mellitus. Circulation. 2004. 110:214–219.24. Odawara M. Nateglinide and mitiglinide. Nippon Rinsho. 2003. 61:1230–1237.25. Ohta Y, Tanizawa Y, Inoue H, Hosaka T, Ueda K, Matsutani A, Repunte VP, Yamada M, Kurachi Y, Bryan J, Aguilar-Bryan L, Permutt MA, Oka Y. Identification and functional analysis of sulfonylurea receptor 1 variants in Japanese patients with NIDDM. Diabetes. 1998. 47:476–481.26. Gribble FM, Tucker SJ, Seino S, Ashcroft FM. Tissue specificity of sulfonylureas: studies on cloned cardiac and beta-cell K(ATP) channels. Diabetes. 1998. 47:1412–1418.27. Miki T, Nagashima K, Seino S. The structure and function of the ATP-sensitive K+channel in insulin-secreting pancreatic beta-cells. J Mol Endocrinol. 1999. 22:113–123.28. Miki T, Nagashima K, Tashiro F, Kotake K, Yoshitomi H, Tamamoto A, Gonoi T, Iwanaga T, Miyazaki J, Seino S. Defective insulin secretion and enhanced insulin action in KATP channel channel channel-deficient mice. Proc Natl Acad Sci U S A. 1998. 95:10402–10406.29. Cole WC, McPherson CD, Sontag D. ATP-regulated K+channels protect the myocardium against ischemia/reperfusion damage. Circ Res. 1991. 69:571–581.30. Terzic A, Jahangir A, Kurachi Y. Cardiac ATP-sensitive K+ channels: regulation by intracellular nucleotides and K+channel-opening drugs. Am J Physiol. 1995. 269:C525–C545.31. Shigematsu S, Sato T, Abe T, Saikawa T, Sakata T, Arita M. Pharmacological evidence for the persistent activation of ATP-sensitive K+channels in early phase of reperfusion and its protective role against myocardial stunning. Circulation. 1995. 92:2266–2275.32. Hu S, Wang S, Dunning BE. Tissue selectivity of antidiabetic agent nateglinide: study on cardiovascular and beta cell K(ATP) channels. J Pharmacol Exp Ther. 1999. 291:1372–1379.33. Quast U, Stephan D, Bieger S, Russ U. The impact of ATP-sensitive K+channel subtype selectivity of insulin secretagogues for the coronary vasculature and the myocardium. Diabetes. 2004. 53:Suppl 3. S156–S164.34. Ojima K, Kiyono Y, Kojima M. Pharmacological and clinical profile of mitiglinide calcium hydrate (Glufast), a new insulinotropic agent with rapid onset. Nippon Yakurigaku Zasshi. 2004. 124:245–255.35. Sunaga Y, Gonoi T, Shibasaki T, Ichikawa K, Kusama H, Yano H, Seino S. The effects of mitiglinide (KAD-1229), a new anti-diabetic drug, on ATP-sensitive K+channels and insulin secretion: comparison with the sulfonylureas and nateglinide. Eur J Pharmacol. 2001. 431:119–125.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Efficacy and Safety of beraprost sodium in Type 2 Diabetic Subjects Complicated with Arteriosclerosis Obliterans (ASO): A Prospective, Multicenter, Open Clinical Study
- Safety, efficacy, and onset of a novel botulinum toxin type A (Nabota) for the treatment of glabellar frown lines: a single-arm, prospective, phase 4 clinical study
- Efficacy and Safety of Botulinum Toxin Type A (NABOTA) for Post-stroke Upper Extremity Spasticity: A Multicenter Phase IV Trial
- Efficacy and Safety of Glimepiride: A Novel Sulfonylurea Drug compared with Gliclazide in the Treatment of Type 2 Diabetes Mellitus: an Open , Randomized Comparative Multi - Center Clinical Study
- RE: Efficacy and Safety of Radiofrequency Ablation for Benign Thyroid Nodules: A Prospective Multicenter Study