J Korean Soc Radiol.
2013 Jul;69(1):57-65. 10.3348/jksr.2013.69.1.57.
Usefulness of Tissue Permeability Factor in Differentiating Benign and Malignant Pulmonary Lesions on Dynamic Contrast-Enhanced MRI
- Affiliations
-
- 1Department of Radiology, Chonbuk National University Hospital, Jeonju, Korea. gyjin@chonbuk.ac.kr
- 2Department of Radiology, Chonbuk National University Medical School and Hospital, Research Institute of Clinical Medicine, Jeonju, Korea.
- 3Department of Internal Medicine, Chonbuk National University Medical School and Hospital, Research Institute of Clinical Medicine, Jeonju, Korea.
- 4Department of Preventive Medicine, Chonbuk National University Medical School and Hospital, Research Institute of Clinical Medicine, Jeonju, Korea.
- KMID: 2002891
- DOI: http://doi.org/10.3348/jksr.2013.69.1.57
Abstract
- PURPOSE
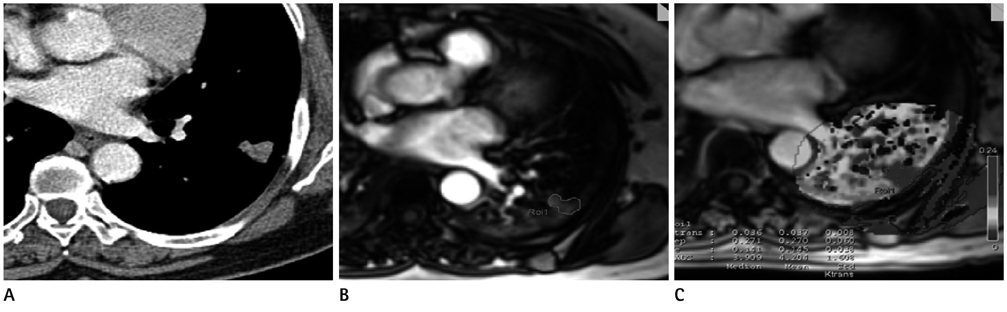
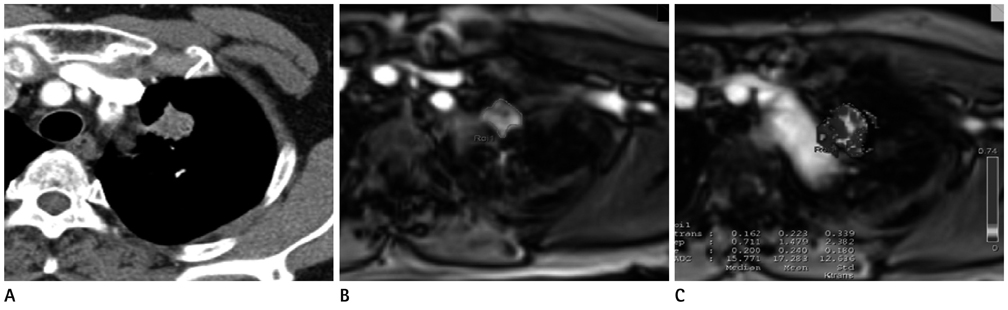
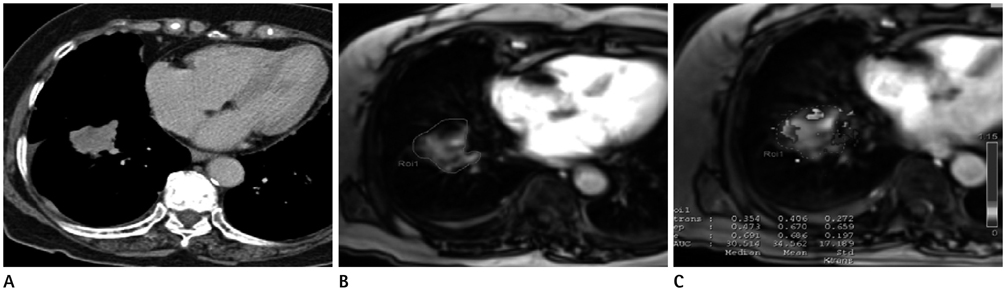
To evaluate the clinical usefulness of tissue permeability factor in differentiating benign and malignant pulmonary lesions on dynamic contrast-enhanced (DCE) MRI.
MATERIALS AND METHODS
30 patients (14 women, 16 men; median age, 64 years; age range, 41-80 years) who had a pulmonary lesion underwent DCE MR imaging at 3.0 T. Fifteen patients had lung cancer and 15 patients had benign pulmonary nodules. To calculate the perfusion parameters of the pulmonary lesions, quantitative analysis was carried out on all 30 pulmonary nodules or masses: volume transfer constant (K(trans)), reflux constant (K(ep)), and extravascular extracellular space volume fraction (v(e)). A Mann-Whitney test was used to calculate the statistical significance of quantitative perfusion parameters between malignant and benign pulmonary lesions. Receiver operating characteristic curve analysis was also performed for evaluation of sensitivity and specificity of perfusion parameters to diagnose lung cancer.
RESULTS
Malignant pulmonary lesions had higher K(trans) and v(e) values than benign pulmonary lesions (0.227 +/- 0.065 vs. 0.133 +/- 0.054; p = 0.001, 0.479 +/- 0.156 vs. 0.357 +/- 0.13; p = 0.038, respectively). However, the difference in K(ep) between the benign and malignant pulmonary lesion was not significant (0.648 +/- 0.44 vs. 0.797 +/- 0.93; p = 0.709). With a threshold of 0.202 (min-1), the sensitivity and specificity to diagnose malignant pulmonary lesions of the K(trans) value were 66.6% and 93.3%, respectively.
CONCLUSION
K(trans) and v(e) value of perfusion parameters on DCE-MRI can help to discriminate between malignant and benign pulmonary nodules or masses.
MeSH Terms
Figure
Reference
-
1. Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA. 2001; 285:914–924.2. Ohno Y, Koyama H, Matsumoto K, Onishi Y, Takenaka D, Fujisawa Y, et al. Differentiation of malignant and benign pulmonary nodules with quantitative first-pass 320-detector row perfusion CT versus FDG PET/CT. Radiology. 2011; 258:599–609.3. Oostendorp M, Post MJ, Backes WH. Vessel growth and function: depiction with contrast-enhanced MR imaging. Radiology. 2009; 251:317–335.4. Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997; 7:91–101.5. Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, et al. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999; 10:223–232.6. O'Connor JP, Jackson A, Asselin MC, Buckley DL, Parker GJ, Jayson GC. Quantitative imaging biomarkers in the clinical development of targeted therapeutics: current and future perspectives. Lancet Oncol. 2008; 9:766–776.7. Marcus CD, Ladam-Marcus V, Cucu C, Bouché O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009; 72:217–238.8. Akisik MF, Sandrasegaran K, Bu G, Lin C, Hutchins GD, Chiorean EG. Pancreatic cancer: utility of dynamic contrast-enhanced MR imaging in assessment of antiangiogenic therapy. Radiology. 2010; 256:441–449.9. Padhani AR. Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging. 2002; 16:407–422.10. Padhani AR, Hayes C, Assersohn L, Powles T, Makris A, Suckling J, et al. Prediction of clinicopathologic response of breast cancer to primary chemotherapy at contrast-enhanced MR imaging: initial clinical results. Radiology. 2006; 239:361–374.11. Kono R, Fujimoto K, Terasaki H, Müller NL, Kato S, Sadohara J, et al. Dynamic MRI of solitary pulmonary nodules: comparison of enhancement patterns of malignant and benign small peripheral lung lesions. AJR Am J Roentgenol. 2007; 188:26–36.12. Pauls S, Mottaghy FM, Schmidt SA, Krüger S, Möller P, Brambs HJ, et al. Evaluation of lung tumor perfusion by dynamic contrast-enhanced MRI. Magn Reson Imaging. 2008; 26:1334–1341.13. Schaefer JF, Vollmar J, Schick F, Vonthein R, Seemann MD, Aebert H, et al. Solitary pulmonary nodules: dynamic contrast-enhanced MR imaging--perfusion differences in malignant and benign lesions. Radiology. 2004; 232:544–553.14. Pauls S, Breining T, Muche R, Schmidt SA, Wunderlich A, Krüger S, et al. The role of dynamic, contrast-enhanced MRI in differentiating lung tumor subtypes. Clin Imaging. 2011; 35:259–265.15. Kaiser WA, Zeitler E. MR imaging of the breast: fast imaging sequences with and without Gd-DTPA. Preliminary observations. Radiology. 1989; 170(3 Pt 1):681–686.16. van der Woude HJ, Verstraete KL, Hogendoorn PC, Taminiau AH, Hermans J, Bloem JL. Musculoskeletal tumors: does fast dynamic contrast-enhanced subtraction MR imaging contribute to the characterization? Radiology. 1998; 208:821–828.17. Barentsz JO, Engelbrecht M, Jager GJ, Witjes JA, de LaRosette J, van Der Sanden BP, et al. Fast dynamic gadolinium-enhanced MR imaging of urinary bladder and prostate cancer. J Magn Reson Imaging. 1999; 10:295–304.18. Jager GJ, Ruijter ET, van de Kaa CA, de la Rosette JJ, Oosterhof GO, Thornbury JR, et al. Local staging of prostate cancer with endorectal MR imaging: correlation with histopathology. AJR Am J Roentgenol. 1996; 166:845–852.19. Liney GP, Turnbull LW, Knowles AJ. In vivo magnetic resonance spectroscopy and dynamic contrast enhanced imaging of the prostate gland. NMR Biomed. 1999; 12:39–44.20. Williams TC, DeMartini WB, Partridge SC, Peacock S, Lehman CD. Breast MR imaging: computer-aided evaluation program for discriminating benign from malignant lesions. Radiology. 2007; 244:94–103.21. Bloch BN, Lenkinski RE, Rofsky NM. The role of magnetic resonance imaging (MRI) in prostate cancer imaging and staging at 1.5 and 3 Tesla: the Beth Israel Deaconess Medical Center (BIDMC) approach. Cancer Biomark. 2008; 4:251–262.22. George ML, Dzik-Jurasz AS, Padhani AR, Brown G, Tait DM, Eccles SA, et al. Non-invasive methods of assessing angiogenesis and their value in predicting response to treatment in colorectal cancer. Br J Surg. 2001; 88:1628–1636.23. Jager GJ, Ruijter ET, van de Kaa CA, de la Rosette JJ, Oosterhof GO, Thornbury JR, et al. Dynamic TurboFLASH subtraction technique for contrast-enhanced MR imaging of the prostate: correlation with histopathologic results. Radiology. 1997; 203:645–652.24. Yao WW, Zhang H, Ding B, Fu T, Jia H, Pang L, et al. Rectal cancer: 3D dynamic contrast-enhanced MRI; correlation with microvascular density and clinicopathological features. Radiol Med. 2011; 116:366–374.25. Ng CS, Raunig DL, Jackson EF, Ashton EA, Kelcz F, Kim KB, et al. Reproducibility of perfusion parameters in dynamic contrast-enhanced MRI of lung and liver tumors: effect on estimates of patient sample size in clinical trials and on individual patient responses. AJR Am J Roentgenol. 2010; 194:W134–W140.26. Bali MA, Metens T, Denolin V, Delhaye M, Demetter P, Closset J, et al. Tumoral and nontumoral pancreas: correlation between quantitative dynamic contrast-enhanced MR imaging and histopathologic parameters. Radiology. 2011; 261:456–466.27. Galbraith SM, Lodge MA, Taylor NJ, Rustin GJ, Bentzen S, Stirling JJ, et al. Reproducibility of dynamic contrast-enhanced MRI in human muscle and tumours: comparison of quantitative and semi-quantitative analysis. NMR Biomed. 2002; 15:132–142.28. Aerts HJ, van Riel NA, Backes WH. System identification theory in pharmacokinetic modeling of dynamic contrast-enhanced MRI: influence of contrast injection. Magn Reson Med. 2008; 59:1111–1119.29. Yu CW, Shih TT, Hsu CY, Lin LC, Wei SY, Lee CM, et al. Correlation between pancreatic microcirculation and type 2 diabetes in patients with coronary artery disease: dynamic contrast-enhanced MR imaging. Radiology. 2009; 252:704–711.30. Kim H, Folks KD, Guo L, Stockard CR, Fineberg NS, Grizzle WE, et al. DCE-MRI detects early vascular response in breast tumor xenografts following anti-DR5 therapy. Mol Imaging Biol. 2011; 13:94–103.31. Ocak I, Bernardo M, Metzger G, Barrett T, Pinto P, Albert PS, et al. Dynamic contrast-enhanced MRI of prostate cancer at 3 T: a study of pharmacokinetic parameters. AJR Am J Roentgenol. 2007; 189:849.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Preliminary Experience Using Dynamic MRI at 3.0 Tesla for Evaluation of Soft Tissue Tumors
- Dynamic Contrast-Enhanced MRI and Its Applications in Various Central Nervous System Diseases
- Clinical Efficacy of Ultrafast Dynamic Contrast-Enhanced MRI Using Compressed Sensing in Distinguishing Benign and Malignant Soft-Tissue Tumors
- Correlation of Contrast-Enhanced MR Findings and Tumor Microvessel Density of Breast Masses
- Usefulness of Dynamic MR Imaging for the Evaluation of the Solitary Pulmonary Nodules Smaller than 15 mm: Differentiation between Benign and Malignant Nodules