J Korean Soc Magn Reson Med.
2011 Aug;15(2):130-138. 10.13104/jksmrm.2011.15.2.130.
Functional MRI of Language: Difference of its Activated Areas and Lateralization according to the Input Modality
- Affiliations
-
- 1Department of Radiology, Gyeongsang National University School of Medicine, Korea. jmcho@gnu.ac.kr
- 2Department of Radiology, Samsung Medical Center, Korea.
- 3Department of Radiology, Kangwon National University College of Medicine, Korea.
- 4Department of Radiology Technology, Eulji University College of Health Sciences, Korea.
- KMID: 2000061
- DOI: http://doi.org/10.13104/jksmrm.2011.15.2.130
Abstract
- PURPOSE
To compare fMRIs of visual and auditory word generation tasks, and to evaluate the difference of its activated areas and lateralization according to the mode of stimuli.
MATERIALS AND METHODS
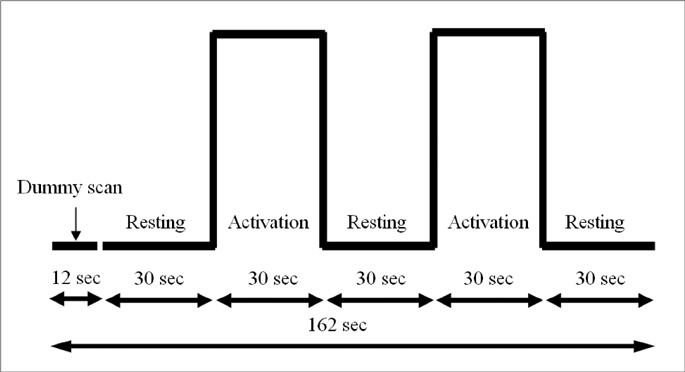
Eight male normal volunteers were included and all were right handed. Functional maps were obtained during auditory and visual word generation tasks in all. Normalized group analysis were performed in each task and the threshold for significance was set at p<0.05. Activated areas in each task were compared visually and statistically.
RESULTS
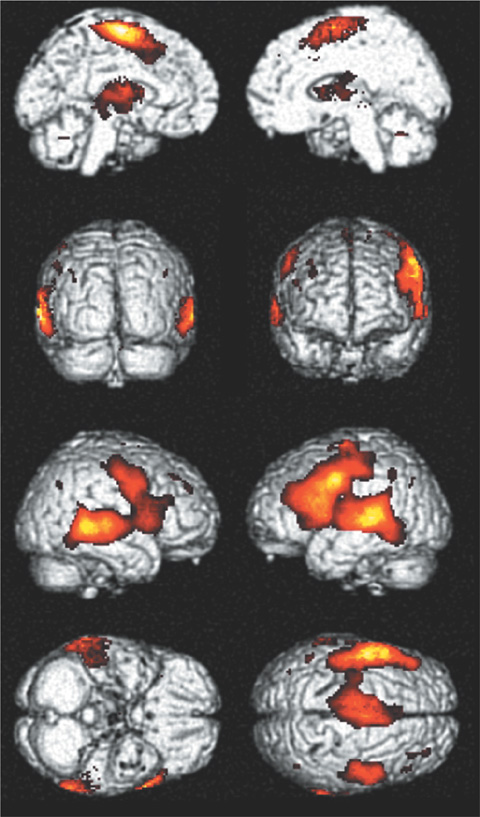
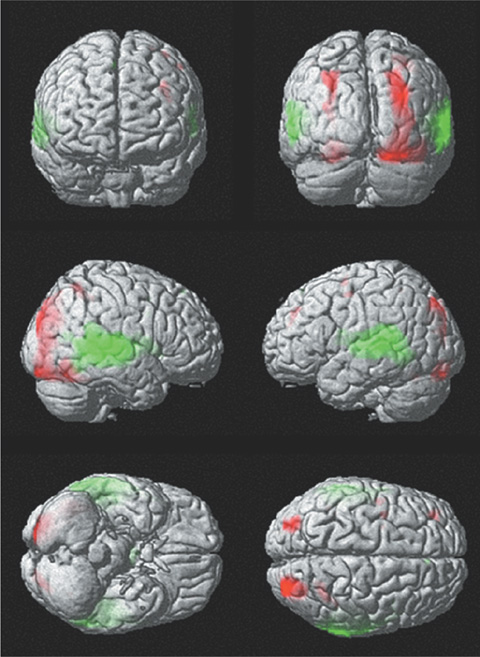
In both tasks, left dominant activations were demonstrated and were more lateralized in visual task. Both frontal lobes (Broca's area, premotor area, and SMA) and left posterior middle temporal gyrus were activated in both tasks. Extensive bilateral temporal activations were noted in auditory task. Both occipital and parietal activations were demonstrated in visual task.
CONCLUSION
Modality independent areas could be interpreted as a core area of language function. Modality specific areas may be associated with processing of stimuli. Visual task induced more lateralized activation and could be a more useful in language study than auditory task.
Keyword
Figure
Reference
-
1. Lichtheim L. On aphasia. Brain. 1885. 7:433–484.2. Geshwind N. Disconnexion syndromes in animals and man. I. Brain. 1965. 88:237–294.3. Rothwell JC, Day BF, Thompson PD. Some experiences of techniques for stimulation of human cerebral motor cortex through the scalp. Neurosurgery. 1987. 20:156–163.4. Toga Arthur, Mazziota John. Brain mapping: the system. 1997. Academic press;301–332. Chapter 14.5. Schaffler L, Luders HO, Dinner DS, Lesser RP, Chelune GJ. Comprehension deficits elicited by electrical stimulation of Brocas area. Brain. 1993. 116:695–715.6. Ojemann GA. Cortical organization of language. J Neurosci. 1991. 11:2281–2287.7. McClelland JL, Rumelhart DE. An interactive Activation model of context effects in letter perception: an account of basic findings. Psychol Rev. 1981. 88:375–407.8. Levelt WJM. Speaking: from intention to articulation. 1997. Cambridge: MIT press.9. Seidenberg MS, McClelland JL. A distributed, developmental model of word recognition and naming. Psychol Rev. 1989. 96:523–568.10. Marshall JC, Newcombe F. Patterns of paralexia: a psycholinguistic approach. J Psycholinguist Res. 1973. 2:175–199.11. McCarthy G, Blamire AM, Rothman DL, Gruetter R, Shulman RG. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proc Natl Acad Sci. 1993. 90:4952–4956.12. Binder J. Functional magnetic resonance imaging: language mapping. Neurosurg Clin N Am. 1997. 8:383–392.13. Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997. 17:353–362.14. Jung HY, Kim JH, Shin T, Piao XH, Kim JS, Lee GK, Park IS, Park JH, Kang SJ, You JJ, Chung SH. Human Brain Mapping of Language-Related Function on 1.5T Magnetic Resonance System: Focused on Motor Language Function. J Korean Radiol Soc. 1998. 38:205–210.15. Yu IK, Chang KH, Song IC, Kim HD, Seong SO, Lee SK, Jang H, Han MH. fMRI of the Motor Speech Center Using EPI. J Korean Radiol Soc. 1998. 38:957–964.16. Ryoo JW, Na DG, Byun HS, Ro DW, Cho JM, Moon CH, Na DL, Chang KH. Functional MRI of Language Area. J Korean Soc Magn Reson Med. 1999. 3:53–59.17. Yoo IR, Ahn KJ, Kim T, Lee JM. Determination of Hemispheric Language Dominance Using Functional MRI: Comparison of Visual and Auditory Stimuli. J Korean Radiol Soc. 1999. 41:1085–1090.18. Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies for the cortical anatomy of single word processing. Nature. 1988. 331:585–589.19. Oldfield RC. The assessment and analysis of handedness: the edinburgh inventory. Neuropsychologia. 1971. 9:97–113.20. Frost JA, Binder JR, Possing ET, Bellgowan TA, Hammeke TA. Stimulus novelty affects superior temporal gyrus activation. Neuroimage. 1998. 7:S377.21. Warburton E, Wise RJS, Price CJ, et al. Noun and verb retrieval by normal subjects studies with PET. Brain. 1996. 119:159–179.22. Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ. A PET study of word finding. Neuropsychologia. 1991. 29:1137–1148.23. Frith CD, Friston KJ, Liddle PF, Frackowiak RSJ. Willed action and the prefrontal cortex in man: a study with PET. Proc R Soc Lond B Biol Sci. 1991. 244:241–246.24. Paulesu E, Frith CD, Frackowiak RS. The neural correlates of the verbal component of working memory. Nature. 1993. 362:342–345.25. Price CJ, Wise RJ, Frackowiak RS. Demonstrating the implicit processing of visually presented words and pseudowords. Cereb Cortex. 1996. 6:62–70.26. Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000. 197:335–359.27. Chee MW, O'Craven KM, Bergida R, Rosen BR, Savoy RL. Auditory and visual word processing studied with fMRI. Hum Brain Mapp. 1999. 7(1):15–28.28. Costello AL, Warrington EK. Dynamic aphasia: the selective impairment of verbal planning. Cortex. 1989. 25:103–114.29. Carpentier A, Pugh KR, Westerveld M, et al. Functional MRI of language processing: dependence on input modality and temporal lobe epilepsy. Epilepsia. 2001. 42:1241–1254.30. Damasio AR, Geschwind N. The neural basis of language. Annu Rev Neurosci. 1984. 7:127–147.31. Mesulam MM. Large-scale neurocognitive networks and distributed processing for attention, language, and memory. Ann Neurol. 1990. 28:597–613.32. Kertesz A, Sheppard A, MacKenzie R. Localization in transcortical sensory aphasia. Arch Neurol. 1982. 39:475–478.33. Alexander MP, Hiltbrunner B, Fischer RS. Distributed anatomy of transcortical sensory aphasia. Arch Neurol. 1989. 46:885–892.34. Hart J Jr, Gordon B. Delineation of single-word semantic comprehension deficits in aphasia, with anatomical correlation. Ann Neurol. 1990. 27:226–231.35. Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991. 114:1803–1817.36. Demonet JF, Chollet F, Ramsay S, et al. The anatomy of phonological and semantic processing in normal subjects. Brain. 1992. 15:1753–1768.37. Damasio H, Grabowski TJ, Tranel D, Hichwa RD, Damasio AR. A neural basis for lexical retrieval. Nature. 1996. 380:499–505.38. Moore CJ, Price CJ. Three distinct ventral occipitotemporal regions for reading and object naming. Neuroimage. 1999. 10:181–192.39. Gorno-Tempini ML, Price CJ, Josephs O, et al. The neural systems sustaining face and proper-name processing. Brain. 1998. 121:2103–2118.40. Mummery CJ, Patterson K, Hodges JR, Price CJ. Functional neuroanatomy of the semantic system: divisible by what? J Cogn Neurosci. 1998. 10:766–777.41. Desmond JE, Sum JM, Wagner AD, et al. Functional MRI measurement of language lateralization in Wada-tested patients. Brain. 1995. 118:1411–1419.42. Binder JR, Swanson SJ, Hammeke TA, et al. Determination of language dominance using functional MRI: a comparison with the Wada test. Neurology. 1996. 46:978–984.43. van der Kallen BFW, Morris GL, Yetkin FZ, van Erning LJTO, Thijssen HOM, Haughton VM. Hemispheric language dominance studied with functional MR: preliminary study in healthy volunteers and patients with epilepsy. AJNR Am J Neuroradiol. 1998. 19:73–77.44. Ryoo JW, Na DG, Byun HS, Moon CH, Shin SW, Kim YH, Paik CH, Ro DW, Kang YW, Hong SB, Kim SM. Language Lateralization by Functional MRI: A Comparison with Wada Test-preliminary Results. J Korean Radiol Soc. 1999. 40:821–827.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Determination of Hemispheric Language Dominance Using Functional MRI: Comparison of Visual and Auditory Stimuli
- Functional MRI Assessment of Hemispheric Language Dominance with Using a Lexical Decision Task
- functional MRI of Language Area
- Localization of motor speech area by functional MRI during word generation
- Brain Language Network and Lateralization Using for Spoken and Written Korean Words in Normal Adults: A Functional MRI Study