Obstet Gynecol Sci.
2015 Jan;58(1):40-45. 10.5468/ogs.2015.58.1.40.
Type-specific persistence or regression of human papillomavirus genotypes in women with cervical intraepithelial neoplasia 1: A prospective cohort study
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Korea University Guro Hospital, Korea University College of Medicine, Seoul, Korea. jhhong93@korea.ac.kr
- KMID: 1994225
- DOI: http://doi.org/10.5468/ogs.2015.58.1.40
Abstract
OBJECTIVE
To evaluate the type-specific human papillomavirus (HPV) persistence or regression in women with or less than low-grade cervical intraepithelial neoplasia (CIN).
METHODS
This prospective cohort study included patients with or less than cytological low-grade squamous intraepithelial lesion (or histologically CIN 1 when biopsy was performed) combined with HPV infection. The cohort was collected from July 2006 to November 2011 at Korea University Guro Hospital. Follow-up was performed with liquid-based Papanicolaou test, hybrid capture 2 test, AnyplexTM II HPV 28 Detection, colposcopic biopsy if necessary every 4 months. All patients were prospectively observed without treatment.
RESULTS
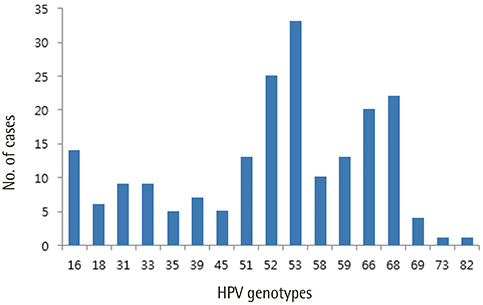
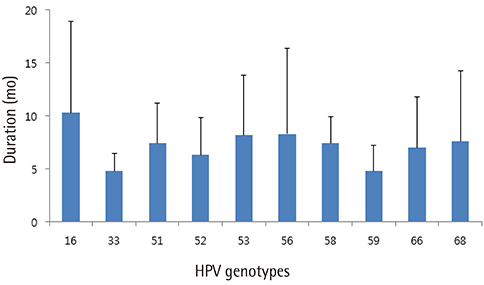
One hundred and thirty-seven patients were enrolled. Of these, 21 patients whose minimum follow-up periods were less than 8 months were excluded. Finally, one hundred sixteen patients were included and followed-up. Median follow-up period was 16 months. In case of high-risk HPVs, HPV 53 was the most prevalent type, followed by HPV 52, 68, 66, and 16. HPV 16 took 10.6 months to regress spontaneously, which was the longest period among the 10 most prevalent high-risk HPV genotypes. In case of spontaneous regression, HPV clearance was always accompanied by lesion clearance. A total of 13 patients showed disease progression either cytologically or histologically. Two cases of CIN 3 were confirmed by colposcopy-directed biopsy during follow-up, which were subsequently managed by conization.
CONCLUSION
HPV 16 is the most persistent HPV genotypes. Studies with longer term follow-up and larger sample size are needed to demonstrate whether persistence of HPV 16 is directly correlated with progression of low-grade lesions.
MeSH Terms
Figure
Cited by 1 articles
-
Risk factors for cytological progression in HPV 16 infected women with ASC-US or LSIL: The Korean HPV cohort
Kyeong A So, Seon Ah Kim, Yoo Kyung Lee, In Ho Lee, Ki Heon Lee, Jee Eun Rhee, Mee Kyung Kee, Chi Heum Cho, Sung Ran Hong, Chang Sun Hwang, Mi Seon Jeong, Ki Tae Kim, Moran Ki, Soo Young Hur, Jong Sup Park, Tae Jin Kim
Obstet Gynecol Sci. 2018;61(6):662-668. doi: 10.5468/ogs.2018.61.6.662.
Reference
-
1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005; 55:74–108.2. Muñoz N, Bosch FX, de Sanjose S, Herrero R, Castellsague X, Shah KV, et al. Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003; 348:518–527.3. Bosch FX, Lorincz A, Muñoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002; 55:244–265.4. Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999; 189:12–19.5. Brown DR, Shew ML, Qadadri B, Neptune N, Vargas M, Tu W, et al. A longitudinal study of genital human papillomavirus infection in a cohort of closely followed adolescent women. J Infect Dis. 2005; 191:182–192.6. Moscicki AB, Shiboski S, Hills NK, Powell KJ, Jay N, Hanson EN, et al. Regression of low-grade squamous intra-epithelial lesions in young women. Lancet. 2004; 364:1678–1683.7. Castellsagué X. Natural history and epidemiology of HPV infection and cervical cancer. Gynecol Oncol. 2008; 110:3 Suppl 2. S4–S7.8. Woodman CB, Collins S, Winter H, Bailey A, Ellis J, Prior P, et al. Natural history of cervical human papillomavirus infection in young women: a longitudinal cohort study. Lancet. 2001; 357:1831–1836.9. Bao YP, Li N, Smith JS, Qiao YL; ACCPAB members. Human papillomavirus type distribution in women from Asia: a meta-analysis. Int J Gynecol Cancer. 2008; 18:71–79.10. Bae JH, Lee SJ, Kim CJ, Hur SY, Park YG, Lee WC, et al. Human papillomavirus (HPV) type distribution in Korean women: a meta-analysis. J Microbiol Biotechnol. 2008; 18:788–794.11. Min KJ, So KA, Lee J, Hong HR, Hong JH, Lee JK, et al. Comparison of the Seeplex HPV4A ACE and the Cervista HPV assays for the detection of HPV in hybrid capture 2 positive media. J Gynecol Oncol. 2012; 23:5–10.12. Estrade C, Sahli R. Comparison of Seegene Anyplex II HPV28 with the PGMY-CHUV assay for human papillomavirus genotyping. J Clin Microbiol. 2014; 52:607–612.13. Smith JS, Lindsay L, Hoots B, Keys J, Franceschi S, Winer R, et al. Human papillomavirus type distribution in invasive cervical cancer and high-grade cervical lesions: a meta-analysis update. Int J Cancer. 2007; 121:621–632.14. Clifford GM, Smith JS, Aguado T, Franceschi S. Comparison of HPV type distribution in high-grade cervical lesions and cervical cancer: a meta-analysis. Br J Cancer. 2003; 89:101–105.15. Clifford GM, Rana RK, Franceschi S, Smith JS, Gough G, Pimenta JM. Human papillomavirus genotype distribution in low-grade cervical lesions: comparison by geographic region and with cervical cancer. Cancer Epidemiol Biomarkers Prev. 2005; 14:1157–1164.16. Kim MJ, Kim JJ, Kim S. Type-specific prevalence of high-risk human papillomavirus by cervical cytology and age: data from the health check-ups of 7,014 Korean women. Obstet Gynecol Sci. 2013; 56:110–120.17. Richardson H, Kelsall G, Tellier P, Voyer H, Abrahamowicz M, Ferenczy A, et al. The natural history of type-specific human papillomavirus infections in female university students. Cancer Epidemiol Biomarkers Prev. 2003; 12:485–490.18. Londesborough P, Ho L, Terry G, Cuzick J, Wheeler C, Singer A. Human papillomavirus genotype as a predictor of persistence and development of high-grade lesions in women with minor cervical abnormalities. Int J Cancer. 1996; 69:364–368.19. Molano M, Van den Brule A, Plummer M, Weiderpass E, Posso H, Arslan A, et al. Determinants of clearance of human papillomavirus infections in Colombian women with normal cytology: a population-based, 5-year follow-up study. Am J Epidemiol. 2003; 158:486–494.20. Woodman CB, Collins SI, Young LS. The natural history of cervical HPV infection: unresolved issues. Nat Rev Cancer. 2007; 7:11–22.21. Mendez F, Munoz N, Posso H, Molano M, Moreno V, van den Brule AJ, et al. Cervical coinfection with human papillomavirus (HPV) types and possible implications for the prevention of cervical cancer by HPV vaccines. J Infect Dis. 2005; 192:1158–1165.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Persistence of HPV after LEEP Treatment in Cervical Intraepithelial Neoplasia
- Effect of human papillomavirus genotype on severity and prognosis of cervical intraepithelial neoplasia
- Effect of Surgical Therapy on the Courses of Human Papillomavirus Infection in Cervical Intraepithelial Neoplasia
- Detection of Human Papillomavius DNA by Hybrid Capture Test in Cervical Intraepithelial Neoplasia and Carcinoma
- The Clinical Significance of the Detection of Human Papilloma Virus in the Patients with ASCUS in the Cervical Pap Smear