Korean J Urol.
2007 Dec;48(12):1308-1314. 10.4111/kju.2007.48.12.1308.
Long-Term Exposure of Rats to a 2.45 GHz Electromagnetic Field: Effects on Reproductive Function
- Affiliations
-
- 1Department of Urology, College of Medicine, Yeungnam University, Daegu, Korea. khmoon@med. yu.ac.kr
- 2Institute of Biomedical Engineering, Yeungnam University, Daegu, Korea.
- KMID: 1990156
- DOI: http://doi.org/10.4111/kju.2007.48.12.1308
Abstract
-
PURPOSE: We wanted to evaluate the effects of a 2.45 GHz electromagnetic field(EMF) radiation on germ cell spermatogenesis.
MATERIALS AND METHODS
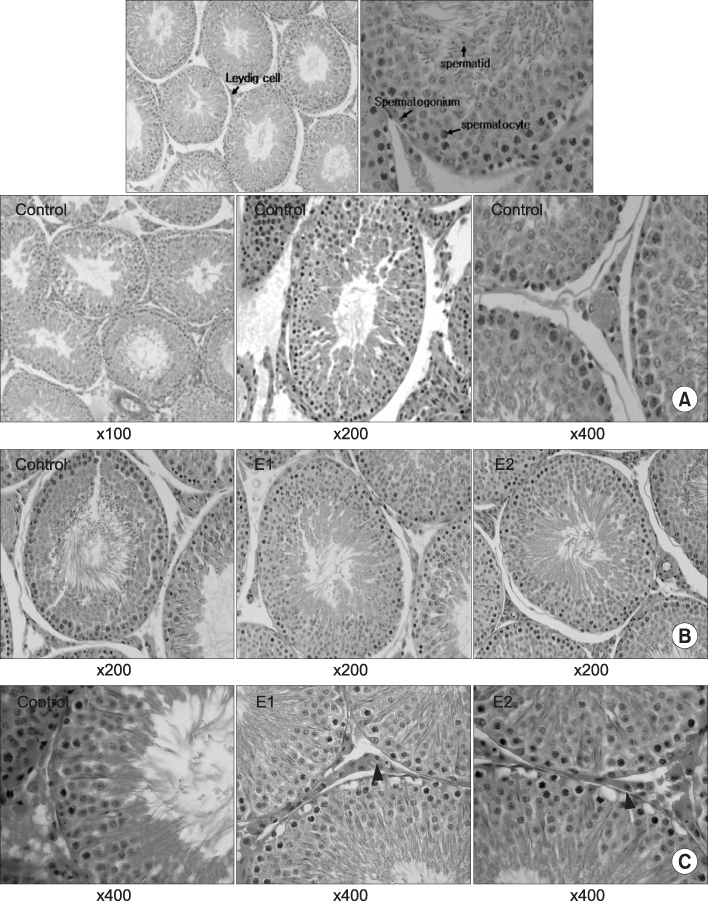
Twenty male Sprague-Dawley rats(4 weeks of age) were exposed to a 2.45 GHz EMF for 1 hour or 2 hours a day. A sham-exposed group served as the control. The whole body average specific absorption rate(SAR) was 1.41 W/kg and the electric field intensity was 60.1mV/m. The rats were confined in cages specially designed for this study, and power was generated by a magnetron. After 8 weeks of exposure, the rats were sacrificed. The testicular germ cell status was assessed by histopathological examination and this was correlated with the hormonal level of the blood serum.
RESULTS
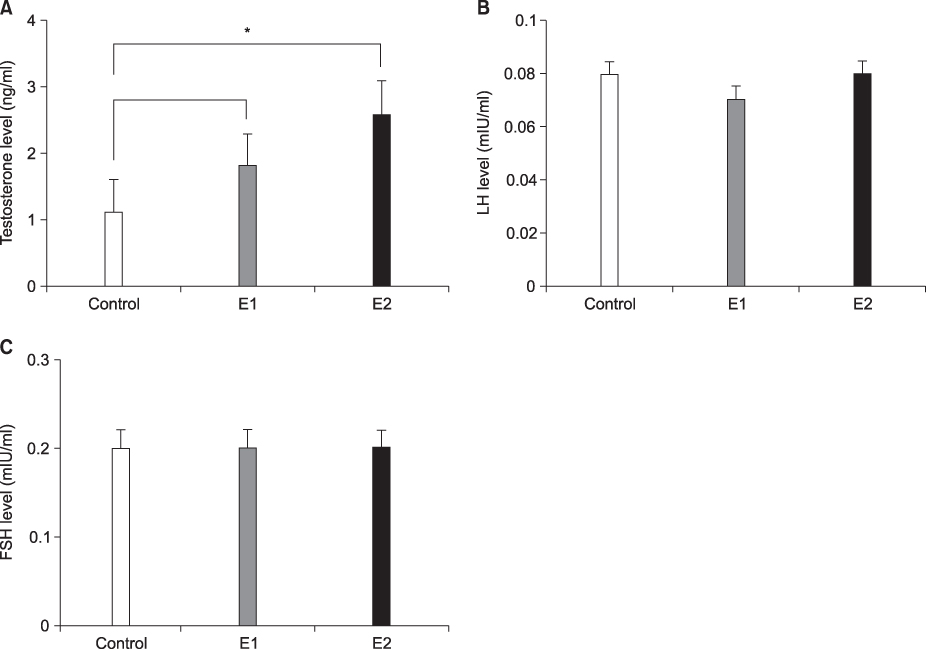
Quantitative analysis of the Leydig cells showed a significantly higher count in the 2 hours exposed rats than in the sham controls(p<0.05), while the difference between the two exposed groups was insignificant. Moreover, a concomitant increase in the serum testosterone level was observed. A significantly decreased number of spermatocytes appeared at the seminiferous tubules in rats exposed for 1 and 2 hours, while this was not seen in the control.
CONCLUSIONS
These changes suggest that long-term exposure to EMF has adverse effects on the proliferation and differentiation of spermatogonia and this may be important in understanding the pathogenesis of EMF- induced male infertility. However, further studies are needed to investigate the effects of a longer exposure time and higher dose.
MeSH Terms
Figure
Reference
-
1. Brent RL. Reproductive and teratologic effects of low-frequency electromagnetic fields: a review of in vivo and in vitro studies using animal models. Teratology. 1999. 59:261–286.2. Kowalczuk CI, Saunders RD, Stapleton HR. Sperm count and sperm abnormality in male mice after exposure to 2.45 GHz microwave radiation. Mutat Res. 1983. 122:155–161.3. Johnsen SG. Testicular biopsy score count - a method for registration of spermatogenesis in human testis: normal values and results in 335 hypogonadal males. Hormones. 1970. 1:2–25.4. Saunders RD, Kowalczuk CI. Effects of 2.45 GHz microwave radiation and heat on mouse spermatogenic epithelium. Int J Radiat Biol Relat Stud Phys Chem Med. 1981. 40:623–632.5. Dewhirst MW, Lora-Michiels M, Viglianti BL, Dewey WC, Repacholi M. Carcinogenic effects of hyperthermia. Int J Hyperthermia. 2003. 19:236–251.6. Blackwell RP. Standards for microwave radiation. Nature. 1979. 282:360.7. Nagler HM, White RD. The effect of testicular torsion on the contralateral testis. J Urol. 1982. 128:1343–1348.8. Hecht K, Balzer HU. Biological effects of electromagnetic fields on humans in the frequency range of 0 to 3 GHz. 1997. Berlin: I.S.F.;1–4.9. Collins P, Lacy D. Studies on the structure and function of the mammalian testis. IV. Steroid metabolism in vitro by isolated interstitium and seminiferous tubules of rat testis after heat sterilization. Proc R Soc Lond B Biol Sci. 1974. 186:37–51.10. Varma MM, Traboulay EA Jr. Biological effects of microwave radiation on the testes of Swiss mice. Experientia. 1975. 31:301–302.11. Meistrich ML. Evaluation of reproductive toxicity by testicular sperm head counts. J Am Coll Toxicol. 1989. 8:551–566.12. Holm M, Rajpert-De Meyts E, Andersson AM, Skakkebaek NE. Leydig cell micronodules are a common finding in testicular biopsies from men with impaired spermatogenesis and are associated with decreased testosterone/LH ratio. J Pathol. 2003. 199:378–386.13. Hand JW, Walker H, Hornsey S, Field SB. Effects of hyperthermia on the mouse testis and its response to X-rays, as assayed by weight loss. Int J Radiat Biol Relat Stud Phys Chem Med. 1979. 35:521–528.14. Singh J, O'Neill C, Handelsman DJ. Induction of spermatogenesis by androgens in gonadotropin-deficient (hpg) mice. Endocrinology. 1995. 136:5311–5321.15. Handelsman DJ, Spaliviero JA, Simpson JM, Allan CM, Singh J. Spermatogenesis without gonadotropins: maintenance has a lower testosterone threshold than initiation. Endocrinology. 1999. 140:3938–3946.16. de Seze R, Fabbro-Peray P, Miro L. GSM radiocellular telephones do not disturb the secretion of antepituitary hormones in humans. Bioelectromagnetics. 1998. 19:271–278.17. Park CY, Nam DS, Kim SH, Shin HJ, Lee JH, Bae JH, et al. Hormonal (cortisol, growth hormone, luteinizing hormone, thyroid stimulating hormone) changes of rabbits exposed to microwaves. J Korean Neurosurg Soc. 1996. 25:920–928.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of electromagnetic field exposure on the reproductive system
- Health effects of electromagnetic fields on children
- Effects of Electromagnetic Radiation Exposure on Stress-Related Behaviors and Stress Hormones in Male Wistar Rats
- The effects of static magnetic field and pulsed electromagnetic field on alkaline phosphatase and dna synthetic activity of ME3t3-E1 cells
- A Study on the Electrophysiological Disturbance of Brain after Electromagnetic Irradiation