Korean J Orthod.
2012 Dec;42(6):307-317. 10.4041/kjod.2012.42.6.307.
Differentiation and characteristics of undifferentiated mesenchymal stem cells originating from adult premolar periodontal ligaments
- Affiliations
-
- 1Department of Orthodontics, School of Dentistry, Pusan National University, Yangsan, Korea. softid@pusan.ac.kr
- 2Department of Oral and Maxillofacial Surgery, School of Dentistry, Pusan National University, Yangsan, Korea.
- 3Department of Orthodontics, School of Dentistry, University of Florida, Florida, USA.
- 4Biomedical Research Institute, Pusan National University Hospital, Busan, Korea.
- KMID: 1976683
- DOI: http://doi.org/10.4041/kjod.2012.42.6.307
Abstract
OBJECTIVE
The purpose of this study was to investigate the isolation and characterization of multipotent human periodontal ligament (PDL) stem cells and to assess their ability to differentiate into bone, cartilage, and adipose tissue.
METHODS
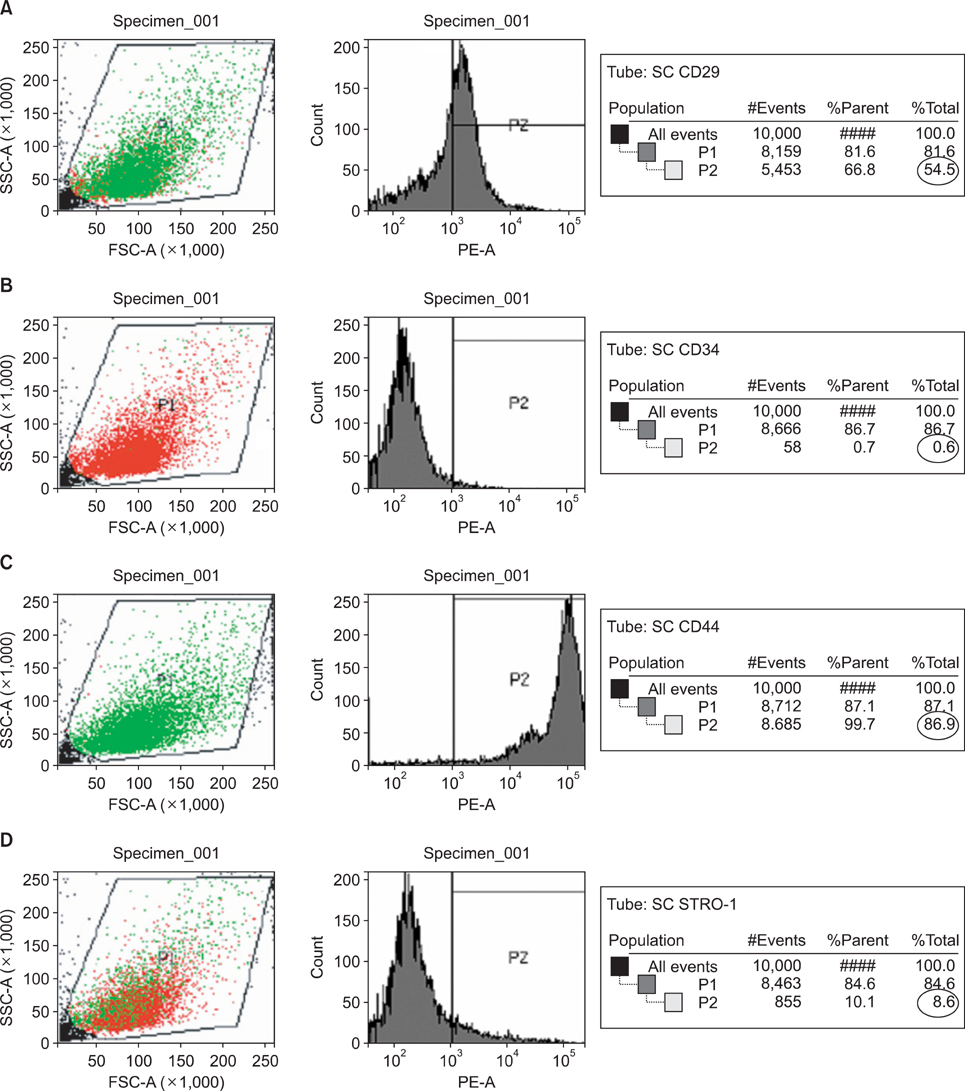
PDL stem cells were isolated from 7 extracted human premolar teeth. Human PDL cells were expanded in culture, stained using anti-CD29, -CD34, -CD44, and -STRO-1 antibodies, and sorted by fluorescent activated cell sorting (FACS). Gingival fibroblasts (GFs) served as a positive control. PDL stem cells and GFs were cultured using standard conditions conducive for osteogenic, chondrogenic, or adipogenic differentiation.
RESULTS
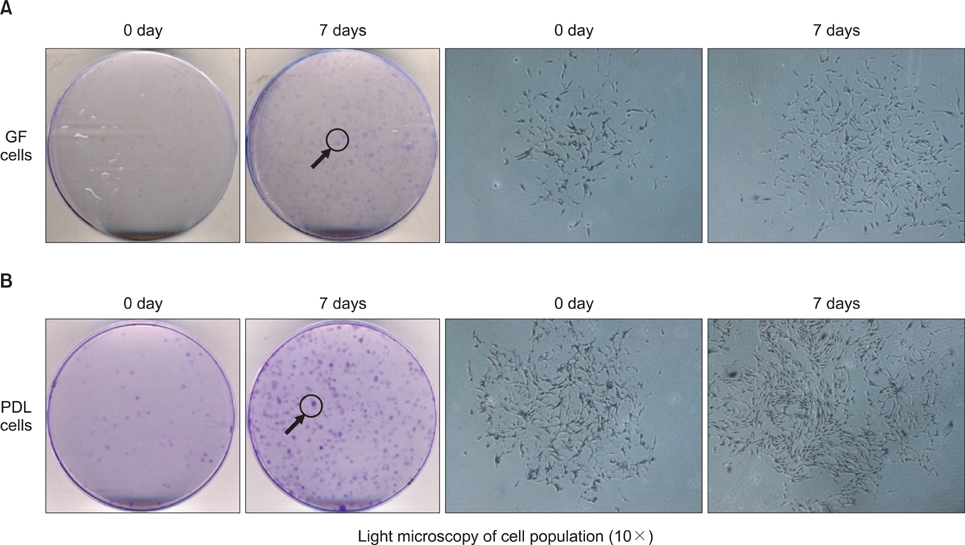
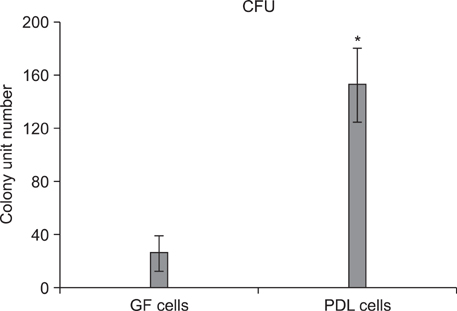
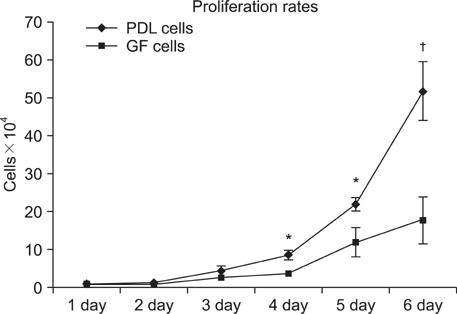
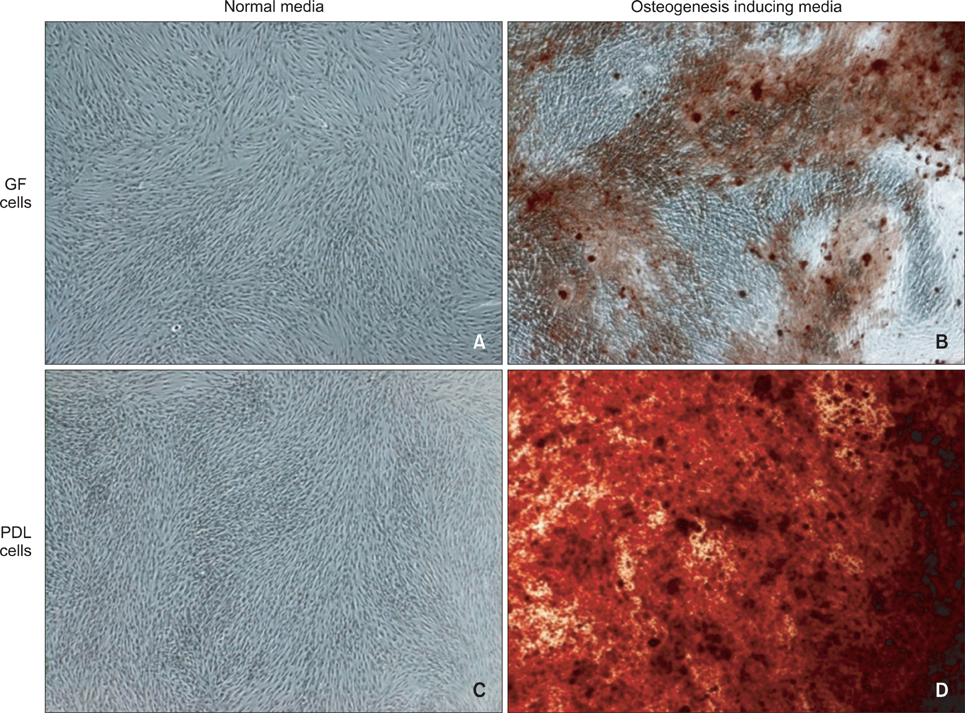
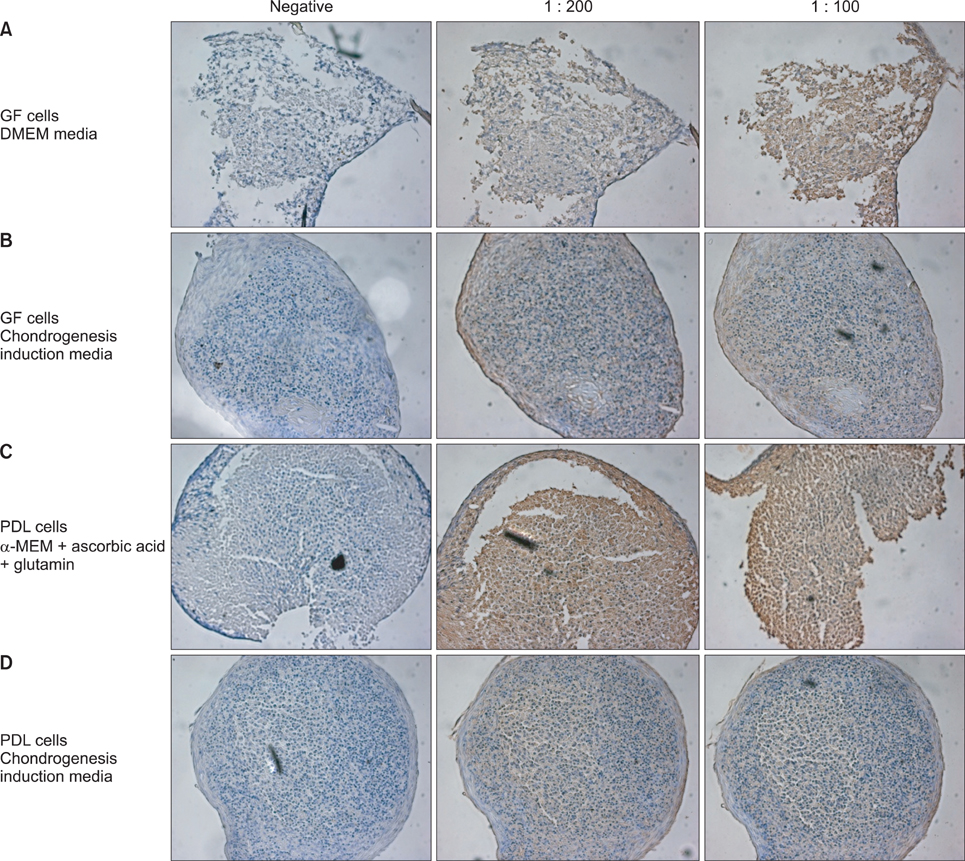
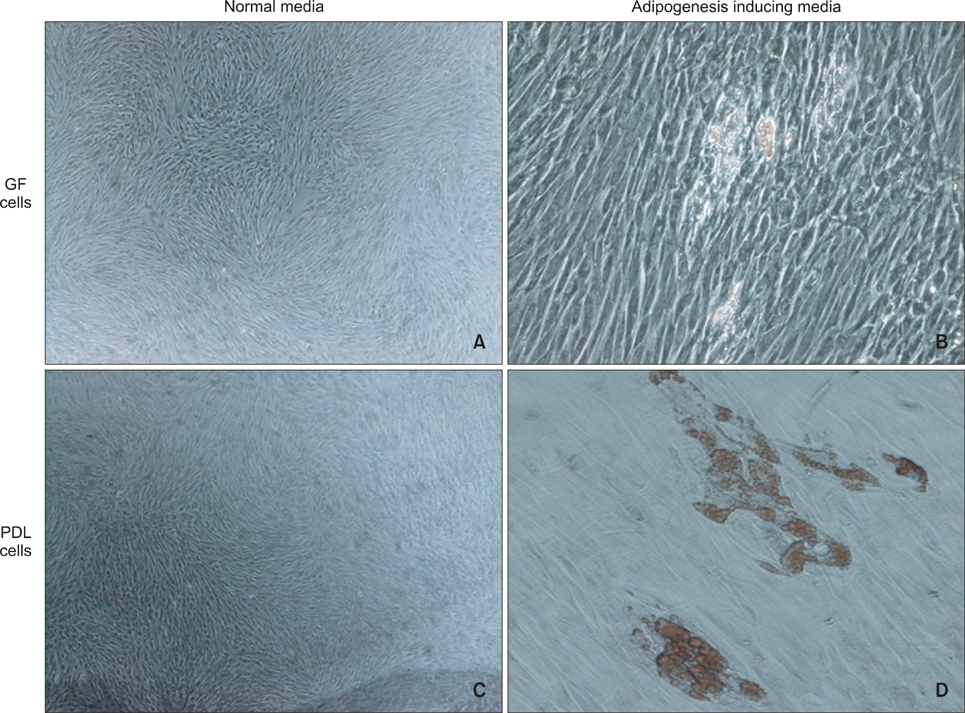
An average of 152.8 +/- 27.6 colony-forming units was present at day 7 in cultures of PDL stem cells. At day 4, PDL stem cells exhibited a significant increase in proliferation (p < 0.05), reaching nearly double the proliferation rate of GFs. About 5.6 +/- 4.5% of cells in human PDL tissues were strongly STRO-1-positive. In osteogenic cultures, calcium nodules were observed by day 21 in PDL stem cells, which showed more intense calcium staining than GF cultures. In adipogenic cultures, both cell populations showed positive Oil Red O staining by day 21. Additionally, in chondrogenic cultures, PDL stem cells expressed collagen type II by day 21.
CONCLUSIONS
The PDL contains multipotent stem cells that have the potential to differentiate into osteoblasts, chondrocytes, and adipocytes. This adult PDL stem cell population can be utilized as potential sources of PDL in tissue engineering applications.
Keyword
MeSH Terms
Figure
Cited by 1 articles
-
Methods of Isolation and Characterization of Stem Cells from Different Regions of Oral Cavity Using Markers: A Systematic Review
Kavarthapu Avinash, Sankari Malaippan, Jayakumar Nadathur Dooraiswamy
Int J Stem Cells. 2017;10(1):12-20. doi: 10.15283/ijsc17010.
Reference
-
1. Friedenstein AJ, Chailakhjan RK, Lalykina KS. The development of fibroblast colonies in monolayer cultures of guinea-pig bone marrow and spleen cells. Cell Tissue Kinet. 1970. 3:393–403.
Article2. Caplan AI. Mesenchymal stem cells. J Orthop Res. 1991. 9:641–650.
Article3. Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999. 284:143–147.
Article4. Alhadlaq A, Mao JJ. Tissue-engineered osteochondral constructs in the shape of an articular condyle. J Bone Joint Surg Am. 2005. 87:936–944.
Article5. Doğan A, Ozdemir A, Kubar A, Oygür T. Healing of artificial fenestration defects by seeding of fibroblast-like cells derived from regenerated periodontal ligament in a dog: a preliminary study. Tissue Eng. 2003. 9:1189–1196.
Article6. Nakahara T, Nakamura T, Kobayashi E, Kuremoto K, Matsuno T, Tabata Y, et al. In situ tissue engineering of periodontal tissues by seeding with periodontal ligament-derived cells. Tissue Eng. 2004. 10:537–544.
Article7. Akizuki T, Oda S, Komaki M, Tsuchioka H, Kawakatsu N, Kikuchi A, et al. Application of periodontal ligament cell sheet for periodontal regeneration: a pilot study in beagle dogs. J Periodontal Res. 2005. 40:245–251.
Article8. Hasegawa M, Yamato M, Kikuchi A, Okano T, Ishikawa I. Human periodontal ligament cell sheets can regenerate periodontal ligament tissue in an athymic rat model. Tissue Eng. 2005. 11:469–478.
Article9. Nussenbaum B, Rutherford RB, Teknos TN, Dornfeld KJ, Krebsbach PH. Ex vivo gene therapy for skeletal regeneration in cranial defects compromised by postoperative radiotherapy. Hum Gene Ther. 2003. 14:1107–1115.
Article10. Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Dis. 2007. 13:151–157.
Article11. Young HE, Duplaa C, Katz R, Thompson T, Hawkins KC, Boev AN, et al. Adult-derived stem cells and their potential for use in tissue repair and molecular medicine. J Cell Mol Med. 2005. 9:753–769.
Article12. Kemp KC, Hows J, Donaldson C. Bone marrow-derived mesenchymal stem cells. Leuk Lymphoma. 2005. 46:1531–1544.
Article13. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 2005. 52:2521–2529.
Article14. Müssig E, Tomakidi P, Steinberg T. Molecules contributing to the maintenance of periodontal tissues. Their possible association with orthodontic tooth movement. J Orofac Orthop. 2005. 66:422–433.
Article15. Krishnan V, Davidovitch Z. Cellular, molecular, and tissue-level reactions to orthodontic force. Am J Orthod Dentofacial Orthop. 2006. 129:469.e1–469.e32.
Article16. Mao JJ, Giannobile WV, Helms JA, Hollister SJ, Krebsbach PH, Longaker MT, et al. Craniofacial tissue engineering by stem cells. J Dent Res. 2006. 85:966–979.
Article17. Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A. 2000. 97:13625–13630.18. Seo BM, Miura M, Gronthos S, Bartold PM, Batouli S, Brahim J, et al. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet. 2004. 364:149–155.
Article19. Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007. 10:149–160.
Article20. Shi S, Bartold PM, Miura M, Seo BM, Robey PG, Gronthos S. The efficacy of mesenchymal stem cells to regenerate and repair dental structures. Orthod Craniofac Res. 2005. 8:191–199.
Article21. Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, et al. Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng. 2007. 13:767–773.
Article22. Laino G, d'Aquino R, Graziano A, Lanza V, Carinci F, Naro F, et al. A new population of human adult dental pulp stem cells: a useful source of living autologous fibrous bone tissue (LAB). J Bone Miner Res. 2005. 20:1394–1402.
Article23. Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A. 2003. 100:5807–5812.
Article24. Digirolamo CM, Stokes D, Colter D, Phinney DG, Class R, Prockop DJ. Propagation and senescence of human marrow stromal cells in culture: a simple colony-forming assay identifies samples with the greatest potential to propagate and differentiate. Br J Haematol. 1999. 107:275–281.
Article25. Reyes M, Lund T, Lenvik T, Aguiar D, Koodie L, Verfaillie CM. Purification and ex vivo expansion of postnatal human marrow mesodermal progenitor cells. Blood. 2001. 98:2615–2625.
Article26. Galotto M, Campanile G, Robino G, Cancedda FD, Bianco P, Cancedda R. Hypertrophic chondrocytes undergo further differentiation to osteoblast-like cells and participate in the initial bone formation in developing chick embryo. J Bone Miner Res. 1994. 9:1239–1249.
Article27. Bennett JH, Joyner CJ, Triffitt JT, Owen ME. Adipocytic cells cultured from marrow have osteogenic potential. J Cell Sci. 1991. 99:131–139.
Article28. Majumdar MK, Thiede MA, Mosca JD, Moorman M, Gerson SL. Phenotypic and functional comparison of cultures of marrow-derived mesenchymal stem cells (MSCs) and stromal cells. J Cell Physiol. 1998. 176:57–66.
Article29. Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood. 1991. 78:55–62.
Article30. Baddoo M, Hill K, Wilkinson R, Gaupp D, Hughes C, Kopen GC, et al. Characterization of mesenchymal stem cells isolated from murine bone marrow by negative selection. J Cell Biochem. 2003. 89:1235–1249.
Article31. Lemoli RM, Tafuri A, Fortuna A, Catani L, Rondelli D, Ratta M, et al. Biological characterization of CD34+ cells mobilized into peripheral blood. Bone Marrow Transplant. 1998. 22:Suppl 5. S47–S50.32. Waller EK, Olweus J, Lund-Johansen F, Huang S, Nguyen M, Guo GR, et al. The "common stem cell" hypothesis reevaluated: human fetal bone marrow contains separate populations of hematopoietic and stromal progenitors. Blood. 1995. 85:2422–2435.
Article33. Sawa Y, Phillips A, Hollard J, Yoshida S, Braithwaite MW. The in vitro life-span of human periodontal ligament fibroblasts. Tissue Cell. 2000. 32:163–170.
Article34. Hou LT, Li TI, Liu CM, Liu BY, Liu CL, Mi HW. Modulation of osteogenic potential by recombinant human bone morphogenic protein-2 in human periodontal ligament cells: effect of serum, culture medium, and osteoinductive medium. J Periodontal Res. 2007. 42:244–252.
Article35. Yamagiwa H, Endo N, Tokunaga K, Hayami T, Hatano H, Takahashi HE. In vivo bone-forming capacity of human bone marrow-derived stromal cells is stimulated by recombinant human bone morphogenetic protein-2. J Bone Miner Metab. 2001. 19:20–28.
Article36. Lecka-Czernik B, Moerman EJ, Grant DF, Lehmann JM, Manolagas SC, Jilka RL. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002. 143:2376–2384.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Skeletal myogenic differentiation of human periodontal ligament stromal cells isolated from orthodontically extracted premolars
- A study on differentiation potency of adult stem cells from pulp, periodontal ligament, and dental follicle to osteoblast
- Adult Mesenchymal Stem Cells for Cell Therapy in Clinical Application
- Stem cell properties of cells derived from canine periodontal ligament