Clin Exp Otorhinolaryngol.
2014 Sep;7(3):210-215. 10.3342/ceo.2014.7.3.210.
The Promoting Effect of Carbamide Peroxide Teeth Bleaching Gel in a Preclinical Model of Head and Neck Cancer in Hamster Buccal Pouch
- Affiliations
-
- 1Laboratory of Medical Skills and Surgical Research, Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Brazil. vfbampi@gmail.com
- 2Department of Food Sciences, Federal University of Lavras, Lavras, Brazil.
- 3Department of Morphology Science, Federal University of Pelotas, Pelotas, Brazil.
- 4Department of Surgery, Faculty of Medicine, Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Brazil.
- KMID: 1973472
- DOI: http://doi.org/10.3342/ceo.2014.7.3.210
Abstract
OBJECTIVES
The aim of this study was to verify the promoting effect of carbamide peroxide on dimethylbenzanthracene (DMBA)-induced carcinogenesis in the hamster buccal pouch, in order to reduce the period of latency for tumor formation.
METHODS
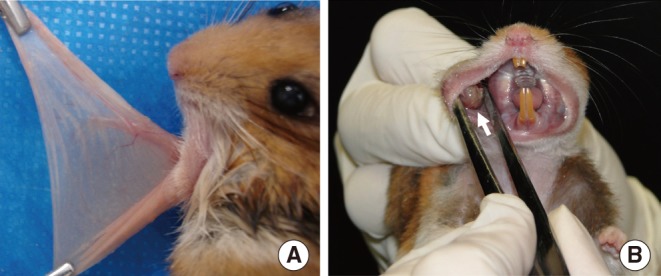
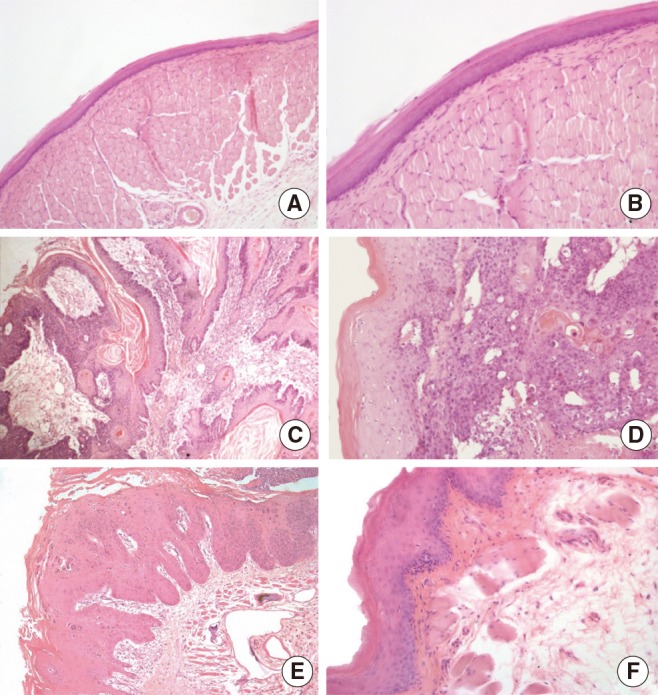
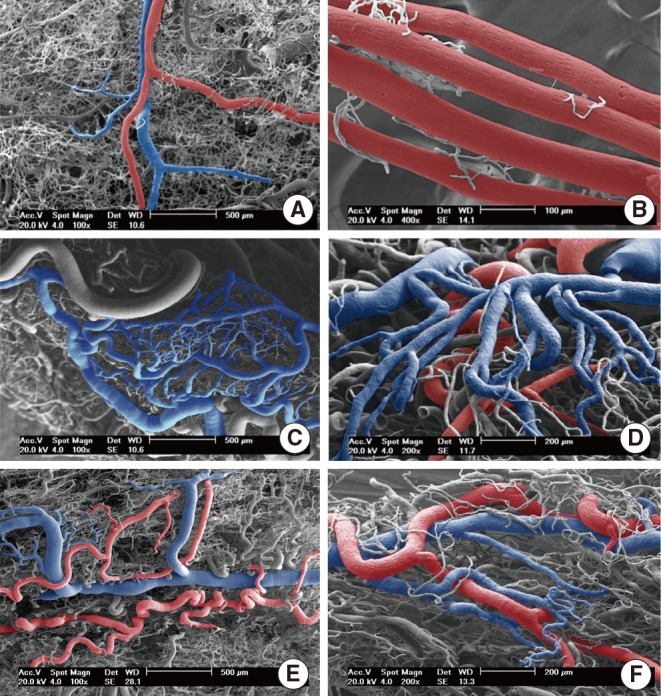
Sixteen hamsters were randomized into two groups of eight animals each. The hamsters of the group I had their right buccal pouches treated with 0.5% DMBA and 10% carbamide peroxide teeth bleaching gel for 55 days. The animals of the group II had their right pouches treated only with DMBA. After, six animals of each group had their pouches prepared for light microscopy. Histomorphometry was performed to assess the presence of keratinization, nuclear polymorphism, pattern of invasion, number of blood vessels, and inflammatory infiltrate in the tumor front. Furthermore, the newly formed lesions were graded according the Bryne's grading system. The remaining animals had the vascular system of the pouches casted by Mercox and qualitatively analyzed by scanning electron microscopy.
RESULTS
Histopathological analysis of the buccal pouches treated with DMBA and carbamide peroxide exhibited formation of squamous cell carcinoma well-differentiated with a high degree of malignancy in all pouches. The development of this neoplasm was associated with a significant increase in the number of blood vessels, presence of keratin pearls, and inflammatory infiltrate. The pouches of the group II showed inflammation, epithelial hyperplasia, dysplasia, and squamous cell carcinoma in only three right pouches. The analysis of the electron micrographs of the pouches chemically inducted with DBMA and carbamide peroxide reveled formation of a new vascular network characteristic of squamous cell carcinoma.
CONCLUSION
The protocol presented here, using DMBA associated with carbamide peroxide, shortens the period of latency to produce squamous cell carcinoma in the hamster buccal pouch, decreasing the time and costs of the experiments.
MeSH Terms
Figure
Reference
-
1. Mognetti B, Di Carlo F, Berta GN. Animal models in oral cancer research. Oral Oncol. 2006; 5. 42(5):448–460. PMID: 16266822.
Article2. Salley JJ. Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res. 1954; 4. 33(2):253–262. PMID: 13152263.
Article3. Chen YK, Lin LM. DMBA-induced hamster buccal pouch carcinoma and VX2-induced rabbit cancer as a model for human oral carcinogenesis. Expert Rev Anticancer Ther. 2010; 9. 10(9):1485–1496. PMID: 20836683.
Article4. Kim S. Animal models of cancer in the head and neck region. Clin Exp Otorhinolaryngol. 2009; 6. 2(2):55–60. PMID: 19565028.
Article5. Morris AL. Factors influencing experimental carcinogensis in the hamster cheek pouch. J Dent Res. 1961; Jan-Feb. 40(1):3–15. PMID: 13772812.6. Heber EM, Monti Hughes A, Pozzi EC, Itoiz ME, Aromando RF, Molinari AJ, et al. Development of a model of tissue with potentially malignant disorders (PMD) in the hamster cheek pouch to explore the long-term potential therapeutic and/or toxic effects of different therapeutic modalities. Arch Oral Biol. 2010; 1. 55(1):46–51. PMID: 19945092.
Article7. Odukoya O, Shklar G. Initiation and promotion in experimental oral carcinogenesis. Oral Surg Oral Med Oral Pathol. 1984; 9. 58(3):315–320. PMID: 6435048.
Article8. Odukoya O, Shklar G. Two-phase carcinogenesis in hamster buccal pouch. Oral Surg Oral Med Oral Pathol. 1982; 11. 54(5):547–552. PMID: 6817251.
Article9. Fasanaro TS. Bleaching teeth: history, chemicals, and methods used for common tooth discolorations. J Esthet Dent. 1992; May-Jun. 4(3):71–78. PMID: 1389350.
Article10. Pohanish RP, Sittig M. Sittig's handbook of toxic and hazardous chemicals and carcinogens. 6th ed. Amsterdam: Elsevier;2012.11. Naik S, Tredwin CJ, Scully C. Hydrogen peroxide tooth-whitening (bleaching): review of safety in relation to possible carcinogenesis. Oral Oncol. 2006; 8. 42(7):668–674. PMID: 16488181.
Article12. Hirota N, Yokoyama T. Enhancing effect of hydrogen peroxide upon duodenal and upper jejunal carcinogenesis in rats. Gann. 1981; 10. 72(5):811–812. PMID: 7327382.13. Ito A, Watanabe H, Naito M, Naito Y. Induction of duodenal tumors in mice by oral administration of hydrogen peroxide. Gann. 1981; 2. 72(1):174–175. PMID: 7274643.14. Weitzman SA, Weitberg AB, Stossel TP, Schwartz J, Shklar G. Effects of hydrogen peroxide on oral carcinogenesis in hamsters. J Periodontol. 1986; 11. 57(11):685–688. PMID: 3104570.
Article15. Institute of Laboratory Animal Resources (US), Committee on Care and Use of Laboratory Animals. Guide for the care and use of laboratory animals. Bethesda, MD: US Department of Health and Human Services, Public Health Service;1996.16. Bryne M. Is the invasive front of an oral carcinoma the most important area for prognostication? Oral Dis. 1998; 6. 4(2):70–77. PMID: 9680893.
Article17. Anneroth G, Batsakis JG, Luna M. Malignancy grading of squamous cell carcinoma in the floor of the mouth related to clinical evaluation. Scand J Dent Res. 1986; 8. 94(4):347–356. PMID: 3462898.
Article18. Lametschwandtner A, Lametschwandtner U, Weiger T. Scanning electron microscopy of vascular corrosion casts--technique and applications: updated review. Scanning Microsc. 1990; 12. 4(4):889–940. PMID: 2094009.19. Konerding MA. Scanning electron microscopy of corrosion casting in medicine. Scanning Microsc. 1991; 9. 5(3):851–865. PMID: 1725561.20. Warnakulasuriya S. Global epidemiology of oral and oropharyngeal cancer. Oral Oncol. 2009; Apr-May. 45(4-5):309–316. PMID: 18804401.
Article21. Ow TJ, Myers JN. Current management of advanced resectable oral cavity squamous cell carcinoma. Clin Exp Otorhinolaryngol. 2011; 3. 4(1):1–10. PMID: 21461056.
Article22. Vairaktaris E, Spyridonidou S, Papakosta V, Vylliotis A, Lazaris A, Perrea D, et al. The hamster model of sequential oral oncogenesis. Oral Oncol. 2008; 4. 44(4):315–324. PMID: 18061531.
Article23. Sullivan SM, Pittman RN. Hamster retractor muscle: a new preparation for intravital microscopy. Microvasc Res. 1982; 5. 23(3):329–335. PMID: 7099023.
Article24. Molinari AJ, Aromando RF, Itoiz ME, Garabalino MA, Monti Hughes A, Heber EM, et al. Blood vessel normalization in the hamster oral cancer model for experimental cancer therapy studies. Anticancer Res. 2012; 7. 32(7):2703–2709. PMID: 22753729.25. Collet AM, Palmieri M, Molinari B, Schwint AE, Itoiz ME. Experimental study to test the potential tumor promotion effect of a tooth bleaching agent. Acta Odontol Latinoam. 2001; 14(1-2):30–34. PMID: 15208934.26. Lin LM, Chen YK, Lai DL, Huang YL. Minimal arecaidine concentrations showing a promotion effect during DMBA-induced hamster cheek pouch carcinogenesis. J Oral Pathol Med. 1996; 2. 25(2):65–68. PMID: 8667258.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Bleaching effect of carbamide peroxide gel on discolored nonvital teeth
- Can carbamide peroxide be as effective as hydrogen peroxide for in-office tooth bleaching and cause less sensitivity? A systematic review
- Effect of vital tooth bleaching agent on dentin bonding
- Evaluation of at-home bleaching protocol with application on different surfaces: bleaching efficacy and hydrogen peroxide permeability
- Effect of dental bleaching on the microhardness and surface roughness of sealed composite resins