Ann Clin Microbiol.
2014 Jun;17(2):29-34. 10.5145/ACM.2014.17.2.29.
Characterization of the Multidrug-Resistant Acinetobacter species Causing a Nosocomial Outbreak at Intensive Care Units in a Korean Teaching Hospital: Suggesting the Correlations with the Clinical and Environmental Samples, Including Respiratory Tract-related Instruments
- Affiliations
-
- 1Department of Laboratory Medicine, Ewha Womans University School of Medicine, Seoul, Korea.
- 2Department of Laboratory Medicine and Research Institute of Bacterial Resistance, Yonsei University College of Medicine, Seoul, Korea. deyong@yuhs.ac
- 3Department of Infection Control, Severance Hospital, Seoul, Korea.
- KMID: 1971091
- DOI: http://doi.org/10.5145/ACM.2014.17.2.29
Abstract
- BACKGROUND
Acinetobacter spp. is an important nosocomial pathogen for which increasing resistance to multiple antimicrobial agents has been observed. Prevalence of multidrug-resistant (MDR) Acinetobacter spp. in the intensive care unit (ICU) at a teaching hospital in Korea started to increase in 2008. The aim of this study was to determine the source of pathogen spread and to characterize the emerging strains at an early stage of outbreak.
METHODS
Samples from respiratory instruments and fomites in the ICUs, as well as from the healthcare workers, were cultured to identify the sources of MDR Acinetobacter spp. Antimicrobial susceptibility was determined by the CLSI disk diffusion method. Pulsed field gel electrophoresis (PFGE) was performed for clinical and environmental isolates in order to determine clonality. Carbapenemase genes were detected by multiplex PCR. Infection control measures including peer-monitoring of hand washing, environmental cleaning and standard precautions were enforced.
RESULTS
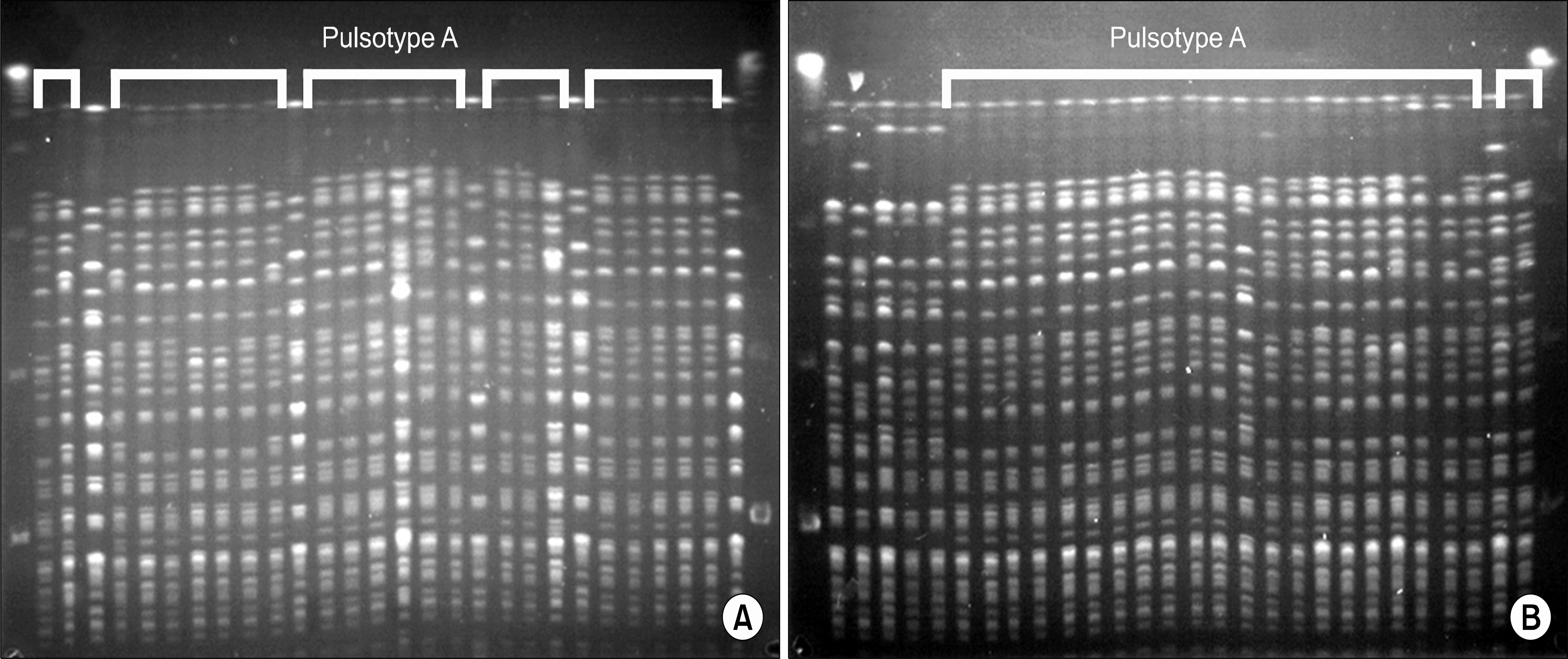
Among the samples from the ICU tools (105) and healthcare worker's hands (44), 31 (30%) and 2 (5%) respective samples yielded MDR Acinetobacter spp. Among the environmental samples, 90% were from respiratory-related equipment. The majority of clinical and environmental MDR Acinetobacter spp. (44/55) belonged to the pulsotype A. baumannii and carried both bla(OXA-51)-like and bla(OXA-23)-like genes. Even though infection-control measures were enforced, prevalence of MDR Acinetobacter spp. continues to increase.
CONCLUSION
An outbreak of MDR Acinetobacter spp. in a Korean hospital was caused by A. baumannii carrying the bla(OXA-23)-gene and was correlated with contaminated respiratory-related instruments in the ICUs. More intensive measures for nosocomial infection control are needed for successful prevention of Acinetobacter spread in hospitals.
MeSH Terms
Figure
Reference
-
1.Lee K., Yong D., Jeong SH., Chong Y. Multidrug-resistant Acinetobacter spp.: increasingly problematic nosocomial pathogens. Yonsei Med J. 2011. 52:879–91.2.Peleg AY., Seifert H., Paterson DL. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev. 2008. 21:538–82.3.Fournier PE., Vallenet D., Barbe V., Audic S., Ogata H., Poirel L, et al. Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genet. 2006. 2:e7.4.Lee K., Lee MA., Lee CH., Lee J., Roh KH., Kim S, et al. KONSAR Group. Increase of ceftazidime- and fluoroquinolone-resistant Klebsiella pneumoniae and imipenem-resistant Acinetobacter spp. in Korea: analysis of KONSAR study data from 2005 and 2007. Yonsei Med J. 2010. 51:901–11.5.Mera RM., Miller LA., Amrine-Madsen H., Sahm DF. Acinetobacter baumannii 2002-2008: increase of carbapenem-associated multiclass resistance in the United States. Microb Drug Resist. 2010. 16:209–15.6.Clinical and Laboratory Standards Institute. Performance Standards for Antimicrobial Susceptibility Testing; Eighteenth Informational Supplement. Document M100-S18. Wanye, PA; Clinical and Laboratory Standards Institute. 2008.7.Lee K., Chong Y., Shin HB., Kim YA., Yong D., Yum JH. Modified Hodge and EDTA-disk synergy tests to screen metallo-beta- lactamase-producing strains of Pseudomonas and Acinetobacter species. Clin Microbiol Infect. 2001. 7:88–91.8.Lee K., Lim YS., Yong D., Yum JH., Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003. 41:4623–9.9.Tenover FC., Arbeit RD., Goering RV., Mickelsen PA., Murray BE., Persing DH, et al. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J Clin Microbiol. 1995. 33:2233–9.
Article10.Lee K., Kim MN., Choi TY., Cho SE., Lee S., Whang DH, et al. KONSAR Group. Wide dissemination of OXA-type carbapenemases in clinical Acinetobacter spp. isolates from South Korea. Int J Antimicrob Agents. 2009. 33:520–4.11.Woodford N., Ellington MJ., Coelho JM., Turton JF., Ward ME., Brown S, et al. Multiplex PCR for genes encoding prevalent OXA carbapenemases in Acinetobacter spp. Int J Antimicrob Agents. 2006. 27:351–3.12.Kim CK., Lee Y., Lee H., Woo GJ., Song W., Kim MN, et al. Prevalence and diversity of carbapenemases among imipenem- nonsusceptible Acinetobacter isolates in Korea: emergence of a novel OXA-182. Diagn Microbiol Infect Dis. 2010. 68:432–8.13.Lee Y., Lee J., Jeong SH., Lee J., Bae IK., Lee K. Carbapenem- non-susceptible Acinetobacter baumannii of sequence type 92 or its single-locus variants with a G428T substitution in zone 2 of the rpoB gene. J Antimicrob Chemother. 2011. 66:66–72.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Outbreak of Acinetobacter septicemia in a neonatal intensive care unit
- Multidrug-resistant Acinetobacter baumannii infection in the intensive care unit
- Extreme Drug Resistant Acinetobacter Nosocomial Ventilator-Associated Pneumonia Treated Successfully with Tigecycline and Amikacin in Intensive Care Unit: A Case Report
- Nosocomial Outbreak of Carbapenem-Resistant Acinetobacter baumannii in Intensive Care Units and Successful Outbreak Control Program
- Modified CHROMagar Acinetobacter Medium for Direct Detection of Multidrug-Resistant Acinetobacter Strains in Nasal and Rectal Swab Samples