Diabetes Metab J.
2013 Oct;37(5):375-384. 10.4093/dmj.2013.37.5.375.
Ruboxistaurin for the Treatment of Diabetic Peripheral Neuropathy: A Systematic Review of Randomized Clinical Trials
- Affiliations
-
- 1Clinical Research Unit, Department of Pharmacy Practice, National Institute of Pharmaceutical and Education Research, Mohali, India. dipikabansal079@gmail.com
- 2Clinical Pharmacology and Therapeutics, University of Hertfordshire, Hertfordshire, UK.
- KMID: 1965985
- DOI: http://doi.org/10.4093/dmj.2013.37.5.375
Abstract
- BACKGROUND
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes mellitus. Protein kinase C (PKC) inhibitor's has been thought to be a potential disease modifying drug's in DPN as it slows or reverse neuropathy's progression. To assesses the efficacy and safety of ruboxistaurin on the progression of symptoms, signs, or functional disability in DPN.
METHODS
A systematic review of the literature databases like PubMed, ProQuest, EBSCO, EMBASE, and Cochrane Central was performed up to August 2012. We included randomized controlled trials (RCTs) comparing PKC inhibitor ruboxistaurin (RBX) with control and lasting at least 6 months. Our primary outcome measure was change in neurological examination, measured by neurological total symptom score (NTSS) and vibration detection threshold (VDT). Secondary outcome measures were total quality of life (QoL), skin microvascular blood flow and others.
RESULTS
Six RCTs were included in review. Change in neurological function assessed by NTSS was reported in six studies, out of which significant difference between the RBX and placebo group seen in four studies favouring treatment group while remaining two studies reported no significant difference. VDT was assessed in only one study in which no significant difference seen between RBX and placebo group. Two studies reported significant improvement in QoL data. Safety data was reported in only two studies in which none of side effect was related to RBX.
CONCLUSION
RBX had effects on DPN in some studies, but the evidence is not enough for meta-analysis and firm conclusion.
MeSH Terms
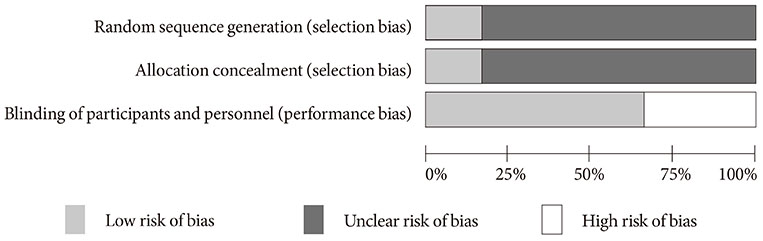
Figure
Reference
-
1. Cabezas-Cerrato J. Neuropathy Spanish Study Group of the Spanish Diabetes Society (SDS). The prevalence of clinical diabetic polyneuropathy in Spain: a study in primary care and hospital clinic groups. Diabetologia. 1998; 41:1263–1269.2. Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, Wilson DM, O'Brien PC, Melton LJ 3rd, Service FJ. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993; 43:817–824.3. Kumar S, Ashe HA, Parnell LN, Fernando DJ, Tsigos C, Young RJ, Ward JD, Boulton AJ. The prevalence of foot ulceration and its correlates in type 2 diabetic patients: a population-based study. Diabet Med. 1994; 11:480–484.4. Young MJ, Boulton AJ, MacLeod AF, Williams DR, Sonksen PH. A multicentre study of the prevalence of diabetic peripheral neuropathy in the United Kingdom hospital clinic population. Diabetologia. 1993; 36:150–154.5. Boulton AJ, Gries FA, Jervell JA. Guidelines for the diagnosis and outpatient management of diabetic peripheral neuropathy. Diabet Med. 1998; 15:508–514.6. Harris M, Eastman R, Cowie C. Symptoms of sensory neuropathy in adults with NIDDM in the U.S. population. Diabetes Care. 1993; 16:1446–1452.7. Edwards JL, Vincent AM, Cheng HT, Feldman EL. Diabetic neuropathy: mechanisms to management. Pharmacol Ther. 2008; 120:1–34.8. Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, Malik RA, Maser RE, Sosenko JM, Ziegler D. American Diabetes Association. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005; 28:956–962.9. Masson EA, Boulton AJ. Aldose reductase inhibitors in the treatment of diabetic neuropathy: a review of the rationale and clinical evidence. Drugs. 1990; 39:190–202.10. Veves A, King GL. Can VEGF reverse diabetic neuropathy in human subjects? J Clin Invest. 2001; 107:1215–1218.11. Chalk C, Benstead TJ, Moore F. Aldose reductase inhibitors for the treatment of diabetic polyneuropathy. Cochrane Database Syst Rev. 2007; (4):CD004572.12. Ramirez MA, Borja NL. Epalrestat: an aldose reductase inhibitor for the treatment of diabetic neuropathy. Pharmacotherapy. 2008; 28:646–655.13. Ring BJ, Gillespie JS, Binkley SN, Campanale KM, Wrighton SA. The interactions of a selective protein kinase C beta inhibitor with the human cytochromes P450. Drug Metab Dispos. 2002; 30:957–961.14. Burkey JL, Campanale KM, Barbuch R, O'Bannon D, Rash J, Benson C, Small D. Disposition of [14C]ruboxistaurin in humans. Drug Metab Dispos. 2006; 34:1909–1917.15. Ohkubo Y, Kishikawa H, Araki E, Miyata T, Isami S, Motoyoshi S, Kojima Y, Furuyoshi N, Shichiri M. Intensive insulin therapy prevents the progression of diabetic microvascular complications in Japanese patients with non-insulin-dependent diabetes mellitus: a randomized prospective 6-year study. Diabetes Res Clin Pract. 1995; 28:103–117.16. Bastyr EJ 3rd, Price KL, Bril V. MBBQ Study Group. Development and validity testing of the neuropathy total symptom score-6: questionnaire for the study of sensory symptoms of diabetic peripheral neuropathy. Clin Ther. 2005; 27:1278–1294.17. Vinik AI, Bril V, Kempler P, Litchy WJ, Tesfaye S, Price KL, Bastyr EJ 3rd. MBBQ Study Group. Treatment of symptomatic diabetic peripheral neuropathy with the protein kinase C beta-inhibitor ruboxistaurin mesylate during a 1-year, randomized, placebo-controlled, double-blind clinical trial. Clin Ther. 2005; 27:1164–1180.18. Bril V. NIS-LL: the primary measurement scale for clinical trial endpoints in diabetic peripheral neuropathy. Eur Neurol. 1999; 41:Suppl 1. 8–13.19. Casellini CM, Barlow PM, Rice AL, Casey M, Simmons K, Pittenger G, Bastyr EJ 3rd, Wolka AM, Vinik AI. A 6-month, randomized, double-masked, placebo-controlled study evaluating the effects of the protein kinase C-beta inhibitor ruboxistaurin on skin microvascular blood flow and other measures of diabetic peripheral neuropathy. Diabetes Care. 2007; 30:896–902.20. Boyd A, Casselini C, Vinik E, Vinik A. Quality of life and objective measures of diabetic neuropathy in a prospective placebo-controlled trial of ruboxistaurin and topiramate. J Diabetes Sci Technol. 2011; 5:714–722.21. Casellini CM, Barlow PM, Rice AL, Casey ME, Simmons K, Pittenger G, Bastyr E, Vinik A. Effect of ruboxistaurin (RBX) on quantitative measures of diabetic peripheral neuropathy (DPN). In : 66th Scientific Session 2006; June 9-13; Washington, DC. Alexandria: American Diabetes Association;2006.22. Casellini CM, Barlow PM, Rice AL, Vinik AA. Effect of ruboxistaurin (RBX) on skin microvascular blood flow (SBF), sensory symptoms, and sensory perception in patients with diabetic peripheral neuropathy (DPN). In : 66th Scientific Session 2006; June 9-13; Washington, DC. Alexandria: American Diabetes Association;2006.23. Brooks B, Delaney-Robinson C, Molyneaux L, Yue DK. Endothelial and neural regulation of skin microvascular blood flow in patients with diabetic peripheral neuropathy: effect of treatment with the isoform-specific protein kinase C beta inhibitor, ruboxistaurin. J Diabetes Complications. 2008; 22:88–95.24. Tesfaye S, Tandan R, Bastyr EJ 3rd, Kles KA, Skljarevski V, Price KL. Ruboxistaurin Study Group. Factors that impact symptomatic diabetic peripheral neuropathy in placebo-administered patients from two 1-year clinical trials. Diabetes Care. 2007; 30:2626–2632.25. Craven PA, Davidson CM, DeRubertis FR. Increase in diacylglycerol mass in isolated glomeruli by glucose from de novo synthesis of glycerolipids. Diabetes. 1990; 39:667–674.26. Hempel A, Maasch C, Heintze U, Lindschau C, Dietz R, Luft FC, Haller H. High glucose concentrations increase endothelial cell permeability via activation of protein kinase C alpha. Circ Res. 1997; 81:363–371.27. Kishimoto A, Takai Y, Mori T, Kikkawa U, Nishizuka Y. Activation of calcium and phospholipid-dependent protein kinase by diacylglycerol, its possible relation to phosphatidylinositol turnover. J Biol Chem. 1980; 255:2273–2276.28. Xia P, Inoguchi T, Kern TS, Engerman RL, Oates PJ, King GL. Characterization of the mechanism for the chronic activation of diacylglycerol-protein kinase C pathway in diabetes and hypergalactosemia. Diabetes. 1994; 43:1122–1129.29. Oates PJ. Aldose reductase, still a compelling target for diabetic neuropathy. Curr Drug Targets. 2008; 9:14–36.30. European Medicines Agency: Withdrawal assessment report for arxxant: Ruboxistaurin (as mesilate monohydrate). updated 2007 May 24. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/Application_withdrawal_assessment_report/2010/01/WC500068826.pdf.31. The DCCT Research Group. Epidemiology of severe hypoglycemia in the diabetes control and complications trial. Am J Med. 1991; 90:450–459.