Clin Exp Vaccine Res.
2015 Jul;4(2):121-129. 10.7774/cevr.2015.4.2.121.
Pneumococcal disease and use of pneumococcal vaccines in Taiwan
- Affiliations
-
- 1Central Regional Center, Centers for Disease Control, Taichung, Taiwan.
- 2Department of Public Health, China Medical University, Taichung, Taiwan.
- 3Center for Research, Diagnostics and Vaccine Development, Centers for Disease Control, Taipei, Taiwan.
- 4Center of General Education, National Taipei University of Nursing and Health Sciences, Taipei, Taiwan.
- 5Molecular Infectious Disease Research Center, Chang Gung Memorial Hospital, Taoyuan, Taiwan. chchiu@adm.cgmh.org.tw
- 6Department of Pediatrics, Chang Gung Memorial Hospital, Chang Gung University, Taoyuan, Taiwan.
- KMID: 1965396
- DOI: http://doi.org/10.7774/cevr.2015.4.2.121
Abstract
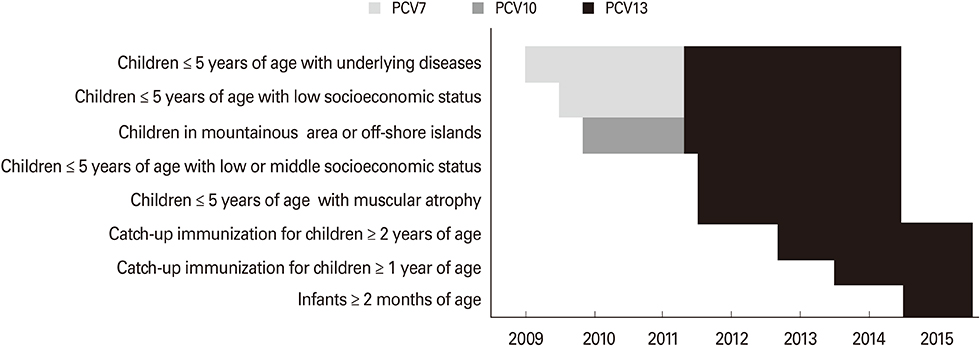
- The use of pneumococcal vaccine plays an important role for prevention of invasive pneumococcal disease (IPD). However, introducing the pneumococcal vaccine into the national immunization program (NIP) is complex and costly. The strategy of progressively integrating the pneumococcal conjugate vaccine (PCV) into the NIP in Taiwan provides valuable experience for policy makers. The 7-valent PCV (PCV7) was first available in Taiwan in late 2005. PCV7 was first provided free to children with underlying diseases, those in vulnerable socioeconomic status, and those with inadequate health care resources. The catch-up immunization program with the 13-valent PCV was launched in 2013 and the national pneumococcal immunization program was implemented in 2015. Children aged 2-5 years had the highest incidence of IPD among pediatric population in Taiwan. Although the incidence of IPD caused by PCV7 serotypes has declined, the overall incidence of IPD remained high in the context of PCV7 use in the private sector. A surge of IPD caused by serotype 19A occurred, accounting for 53.6% of IPD cases among children aged < or = 5 years in 2011-2012. After the implementation of the national pneumococcal immunization program, serogroup 15 has become the leading serogroup for IPD in children. Continued surveillance is necessary to monitor the serotype epidemiology in Taiwan.
Keyword
MeSH Terms
Figure
Reference
-
1. World Health Organization. Immunization, vaccines and biologicals. Estimated Hib and pneumococcal deaths for children under 5 years of age, 2008 [Internet]. Geneva: World Health Organization;2013. cited 2015 Jun 24. Available from: http://www.who.int/immunization/monitoring_surveillance/burden/estimates/Pneumo_hib/en/.2. O'Brien KL, Wolfson LJ, Watt JP, et al. Burden of disease caused by Streptococcus pneumoniae in children younger than 5 years: global estimates. Lancet. 2009; 374:893–902.3. Centers for Disease Control (CDC). Update: pneumococcal polysaccharide vaccine usage, United States. MMWR Morb Mortal Wkly Rep. 1984; 33:273–276. 2814. Progress in introduction of pneumococcal conjugate vaccine worldwide, 2000-2012. Wkly Epidemiol Rec. 2013; 88:173–180.5. Centers for Disease Control and Prevention (CDC). Advisory Committee on Immunization Practices. Updated recommendations for prevention of invasive pneumococcal disease among adults using the 23-valent pneumococcal polysaccharide vaccine (PPSV23). MMWR Morb Mortal Wkly Rep. 2010; 59:1102–1106.6. Advisory Committee on Immunization Practices. Preventing pneumococcal disease among infants and young children. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2000; 49:1–35.7. Nuorti JP, Whitney CG. Centers for Disease Control and Prevention (CDC). Prevention of pneumococcal disease among infants and children: use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2010; 59:1–18.8. Pneumococcal vaccines WHO position paper, 2012. Wkly Epidemiol Rec. 2012; 87:129–144.9. European Medicines Agency. Prevenar, pneumococcal saccharide conjugated vaccine, adsorbed [Internet]. London: European Medicines Agency;2008. cited 2015 Jun 22. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000323/human_med_000987.jsp&%20%20jsenabled=true.10. European Medicines Agency. Prevenar 13, pneumococcal polysaccharide conjugate vaccine (13-valent, adsorbed) [Internet]. London: European Medicines Agency;2010. cited 2015 Jun 15. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/001104/human_med_001220.jsp%20&%20mid=WC0b01ac058001d124.11. European Medicines Agency. Synflorix, pneumococcal polysaccharide conjugate vaccine (adsorbed) [Internet]. London: European Medicines Agency;2009. cited 2015 Jun 15. Available from: http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/000973/human_med_001071.jsp%20&%20mid=WC0b01ac058001d124.12. Meeting of the Strategic Advisory Group of Experts on Immunization, November 2011: conclusions and recommendations. Wkly Epidemiol Rec. 2012; 87:1–16.13. Leal J, Vanderkooi OG, Church DL, Macdonald J, Tyrrell GJ, Kellner JD. Eradication of invasive pneumococcal disease due to the seven-valent pneumococcal conjugate vaccine serotypes in Calgary, Alberta. Pediatr Infect Dis J. 2012; 31:e169–e175.
Article14. Hsu HE, Shutt KA, Moore MR, et al. Effect of pneumococcal conjugate vaccine on pneumococcal meningitis. N Engl J Med. 2009; 360:244–256.
Article15. Kyaw MH, Lynfield R, Schaffner W, et al. Effect of introduction of the pneumococcal conjugate vaccine on drug-resistant Streptococcus pneumoniae. N Engl J Med. 2006; 354:1455–1463.
Article16. Taiwan Food and Drug Administration. Prevenar [Internet]. Taipei: Food and Drug Administration, Ministry of Health and Welfare;2002. cited 2015 Jun 25. Available from: http://www.fda.gov.tw/MLMS/(S(z5rilkfwxazzy1nevt45eza3))/H0001D.aspx?Type=Lic&LicId=10000734.17. Taiwan Food and Drug Administration. Synflorix [Internet]. Taipei: Food and Drug Administration, Ministry of Health and Welfare;2009. cited 2015 Jun 25. Available from: http://www.fda.gov.tw/MLMS/(S(z5rilkfwxazzy1nevt45eza3))/H0001D.aspx?Type=Lic&LicId=10000891.18. Taiwan Food and Drug Administration. Prevenar 13 [Internet]. Taipei: Food and Drug Administration, Ministry of Health and Welfare;2010. cited 2015 Jun 25. Available from: http://www.fda.gov.tw/MLMS/(S(z5rilkfwxazzy1nevt45eza3))/H0001D.aspx?Type=Lic&LicId=10000906.19. Taiwan Food and Drug Administration. Pneumovax 23 [Internet]. Taipei: Food and Drug Administration, Ministry of Health and Welfare;1998. cited 2015 Jun 15. Available from: http://www.fda.gov.tw/MLMS/(S(33hbig2nf0sgwr55vhr3r52x))/H0001D.aspx?Type=Lic&LicId=10000492.20. Liao WH, Lin SH, Lai CC, et al. Impact of pneumococcal vaccines on invasive pneumococcal disease in Taiwan. Eur J Clin Microbiol Infect Dis. 2010; 29:489–492.
Article21. Chang YC, Chou YJ, Liu JY, Yeh TF, Huang N. Additive benefits of pneumococcal and influenza vaccines among elderly persons aged 75 years or older in Taiwan: a representative population-based comparative study. J Infect. 2012; 65:231–238.
Article22. Taiwan Centers for Disease Control. The history of national immunization program [Internet]. Taipei: Taiwan Centers for Disease Control;2015. cited 2015 Mar 15. Available from: http://www.cdc.gov.tw/professional/page.aspx?treeid=5B0231BEB94EDFFC&nowtreeid=C34D70525A597CF9.23. Tsai HY, Chen YH, Liao CH, et al. Trends in the antimicrobial susceptibilities and serotypes of Streptococcus pneumoniae: results from the Tigecycline In Vitro Surveillance in Taiwan (TIST) study, 2006-2010. Int J Antimicrob Agents. 2013; 42:312–316.
Article24. Hsieh YC, Chiu CH, Chang KY, et al. The impact of the heptavalent pneumococcal conjugate vaccine on risk factors for Streptococcus pneumoniae carriage in children. Pediatr Infect Dis J. 2012; 31:e163–e168.
Article25. Pneumococcal conjugate vaccine for childhood immunization: WHO position paper. Wkly Epidemiol Rec. 2007; 82:93–104.26. Chiang CS, Chen YY, Jiang SF, et al. National surveillance of invasive pneumococcal diseases in Taiwan, 2008-2012: differential temporal emergence of serotype 19A. Vaccine. 2014; 32:3345–3349.
Article27. Wei SH, Chiang CS, Chiu CH, Chou P, Lin TY. Pediatric invasive pneumococcal disease in Taiwan following a national catch-up program with the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J. 2015; 34:e71–e77.
Article28. Hsieh YC, Lin PY, Chiu CH, et al. National survey of invasive pneumococcal diseases in Taiwan under partial PCV7 vaccination in 2007: emergence of serotype 19A with high invasive potential. Vaccine. 2009; 27:5513–5518.
Article29. Kuo CY, Hwang KP, Hsieh YC, et al. Nasopharyngeal carriage of Streptococcus pneumoniae in Taiwan before and after the introduction of a conjugate vaccine. Vaccine. 2011; 29:5171–5177.
Article30. Poolman J, Frasch C, Nurkka A, Kayhty H, Biemans R, Schuerman L. Impact of the conjugation method on the immunogenicity of Streptococcus pneumoniae serotype 19F polysaccharide in conjugate vaccines. Clin Vaccine Immunol. 2011; 18:327–336.
Article31. Domingues CM, Verani JR, Montenegro Renoiner EI, et al. Effectiveness of ten-valent pneumococcal conjugate vaccine against invasive pneumococcal disease in Brazil: a matched case-control study. Lancet Respir Med. 2014; 2:464–471.
Article32. Prymula R, Peeters P, Chrobok V, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet. 2006; 367:740–748.
Article33. Kung YH, Chiu NC, Lee KS, et al. Bacterial etiology of acute otitis media in the era prior to universal pneumococcal vaccination in Taiwanese children. J Microbiol Immunol Infect. 2014; 47:239–244.
Article34. Hsueh PR, Teng LJ, Wu TL, et al. Telithromycin- and fluoroquinolone-resistant Streptococcus pneumoniae in Taiwan with high prevalence of resistance to macrolides and beta-lactams: SMART program 2001 data. Antimicrob Agents Chemother. 2003; 47:2145–2151.
Article35. McDonald LC, Lauderdale TL, Shiau YR, et al. The status of antimicrobial resistance in Taiwan among Gram-positive pathogens: the Taiwan Surveillance of Antimicrobial Resistance (TSAR) programme, 2000. Int J Antimicrob Agents. 2004; 23:362–370.
Article36. Tsai HY, Lauderdale TL, Wang JT, et al. Updated antibiotic resistance and clinical spectrum of infections caused by Streptococcus pneumoniae in Taiwan: emphasis on risk factors for penicillin nonsusceptibilities. J Microbiol Immunol Infect. 2013; 46:345–351.
Article37. Kim SH, Song JH, Chung DR, et al. Changing trends in antimicrobial resistance and serotypes of Streptococcus pneumoniae isolates in Asian countries: an Asian Network for Surveillance of Resistant Pathogens (ANSORP) study. Antimicrob Agents Chemother. 2012; 56:1418–1426.
Article38. Safari D, Kuo LC, Huang YT, Liao CH, Sheng WH, Hsueh PR. Increase in the rate of azithromycin-resistant Streptococcus pneumoniae isolates carrying the erm(B) and mef(A) genes in Taiwan, 2006-2010. BMC Infect Dis. 2014; 14:704.
Article39. Lee LH, Chang WN, Huang CR, et al. Adult Streptococcus pneumoniae meningitis in Southern Taiwan: epidemiologic trends and prognostic factors. J Clin Neurosci. 2005; 12:32–35.
Article40. Taiwan Centers for Disease Control. Surveillance projects for invasive pneumococcal disease [Internet]. Taipei: Taiwan Centers for Disease Control;2015. cited 2015 Jun 15. Available from: http://www.cdc.gov.tw/professional/ProgramResult.aspx?did=682&treeid=098f3bc4756f62cc&nowtreeid=A1C23D0C8CDB70DA.41. Chen YY, Yao SM, Chou CY, et al. Surveillance of invasive Streptococcus pneumoniae in Taiwan, 2002-2003. J Med Microbiol. 2006; 55(Pt 8):1109–1114.
Article42. Fung CP, Hu BS, Lee SC, et al. Antimicrobial resistance of Streptococcus pneumoniae isolated in Taiwan: an island-wide surveillance study between 1996 and 1997. J Antimicrob Chemother. 2000; 45:49–55.
Article43. Siu LK, Chu ML, Ho M, Lee YS, Wang CC. Epidemiology of invasive pneumococcal infection in Taiwan: antibiotic resistance, serogroup distribution, and ribotypes analyses. Microb Drug Resist. 2002; 8:201–208.
Article44. Lin WJ, Lo WT, Chou CY, et al. Antimicrobial resistance patterns and serotype distribution of invasive Streptococcus pneumoniae isolates from children in Taiwan from 1999 to 2004. Diagn Microbiol Infect Dis. 2006; 56:189–196.
Article45. Lauderdale TL, Wagener MM, Lin HM, et al. Serotype and antimicrobial resistance patterns of Streptococcus pneumoniae isolated from Taiwanese children: comparison of nasopharyngeal and clinical isolates. Diagn Microbiol Infect Dis. 2006; 56:421–426.
Article46. Hsieh YC, Huang YC, Lin HC, et al. Characterization of invasive isolates of Streptococcus pneumoniae among Taiwanese children. Clin Microbiol Infect. 2009; 15:991–996.
Article47. Chen YY, Yao SM, Chen YH, et al. Antimicrobial susceptibility of invasive Streptococcus pneumoniae in Taiwan, 2008-2012. Taiwan Epidemiol Bull. 2013; 29:232–242.48. Janapatla RP, Hsu MH, Du JF, Hsieh YC, Lin TY, Chiu CH. Sequence types and antimicrobial susceptibility of invasive streptococcus pneumoniae isolates from a region with high antibiotic selective pressure and suboptimal vaccine coverage. Pediatr Infect Dis J. 2010; 29:467–469.
Article49. Shin J, Baek JY, Kim SH, Song JH, Ko KS. Predominance of ST320 among Streptococcus pneumoniae serotype 19A isolates from 10 Asian countries. J Antimicrob Chemother. 2011; 66:1001–1004.
Article50. Choi EH, Kim SH, Eun BW, et al. Streptococcus pneumoniae serotype 19A in children, South Korea. Emerg Infect Dis. 2008; 14:275–281.51. Taiwan Centers for Disease Control. Epidemiological weekly report on invasive pneumococcal disease [Internet]. Taipei: Taiwan Centers for Disease Control;2015. cited 2015 Jun 15. Available from: http://www.cdc.gov.tw/professional/list.aspx?treeid=098f3bc4756f62cc&nowtreeid=90960C331E13A586.52. Chen CJ, Huang YC, Su LH, Lin TY. Nasal carriage of Streptococcus pneumoniae in healthy children and adults in northern Taiwan. Diagn Microbiol Infect Dis. 2007; 59:265–269.
Article53. Chiou CC, Liu YC, Huang TS, et al. Extremely high prevalence of nasopharyngeal carriage of penicillin-resistant Streptococcus pneumoniae among children in Kaohsiung, Taiwan. J Clin Microbiol. 1998; 36:1933–1937.
Article54. Lee NY, Song JH, Kim S, et al. Carriage of antibiotic-resistant pneumococci among Asian children: a multinational surveillance by the Asian Network for Surveillance of Resistant Pathogens (ANSORP). Clin Infect Dis. 2001; 32:1463–1469.
Article55. Lo WT, Wang CC, Yu CM, Chu ML. Rate of nasopharyngeal carriage, antimicrobial resistance and serotype of Streptococcus pneumoniae among children in northern Taiwan. J Microbiol Immunol Infect. 2003; 36:175–181.56. Janapatla RP, Chang HJ, Hsu MH, Hsieh YC, Lin TY, Chiu CH. Nasopharyngeal carriage of Streptococcus pneumoniae, Haemophilus influenzae, Moraxella catarrhalis, and Alloiococcus otitidis in young children in the era of pneumococcal immunization, Taiwan. Scand J Infect Dis. 2011; 43:937–942.
Article57. Hsieh YC, Hsueh PR, Lu CY, Lee PI, Lee CY, Huang LM. Clinical manifestations and molecular epidemiology of necrotizing pneumonia and empyema caused by Streptococcus pneumoniae in children in Taiwan. Clin Infect Dis. 2004; 38:830–835.
Article58. Janapatla RP, Hsu MH, Hsieh YC, Lee HY, Lin TY, Chiu CH. Necrotizing pneumonia caused by nanC-carrying serotypes is associated with pneumococcal haemolytic uraemic syndrome in children. Clin Microbiol Infect. 2013; 19:480–486.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Invasive Pneumococcal Diseases in Korean Adults After the Introduction of Pneumococcal Vaccine into the National Immunization Program
- An Update on Pneumococcal Vaccination
- Indirect Effects of Pneumococcal Conjugate Vaccines in National Immunization Programs for Children on Adult Pneumococcal Disease
- Efficacy and effectiveness of extended-valency pneumococcal conjugate vaccines
- Pneumococcal vaccine