J Cerebrovasc Endovasc Neurosurg.
2014 Sep;16(3):166-174. 10.7461/jcen.2014.16.3.166.
Primary Angioplasty for Symptomatic Atherosclerotic Middle Cerebral Artery Stenosis
- Affiliations
-
- 1Department of Neurosurgery, College of Medicine, Ewha Womans University, Seoul, Korea. nshsg@ewha.ac.kr
- 2Department of Neurosurgery, Seoul National University Bundang Hospital, Seongnam, Korea.
- KMID: 1963153
- DOI: http://doi.org/10.7461/jcen.2014.16.3.166
Abstract
OBJECTIVE
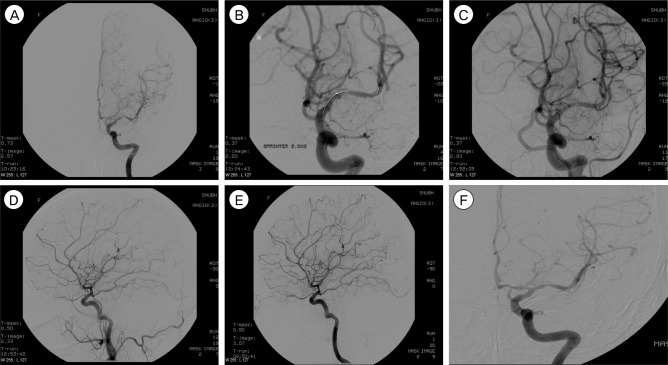
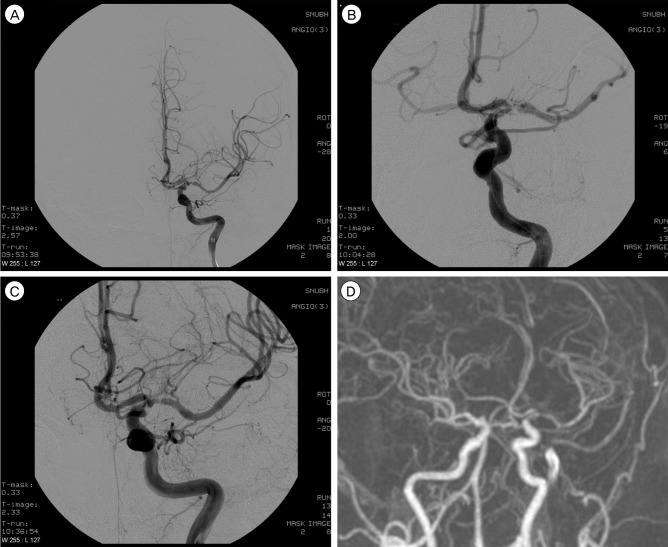
The objective of this study is to evaluate the clinical and angiographic outcomes after primary balloon angioplasty in patients with symptomatic middle cerebral artery (MCA, M1 segment) stenosis refractory to medical therapy.
MATERIALS AND METHODS
Eleven patients with intracranial stenosis were treated with primary balloon angioplasty. All patients had MCA stenosis with recurrent transient ischemic attack (TIA). The indication for balloon angioplasty was patients with significant MCA stenosis: 1) age older than 18 years with recurrent or progressive TIA or infarction despite optimal medical therapy, including anti-coagulation, dual anti-platelet, and anti-lipid medication; 2) previous ischemic events or asymptomatic severe stenosis (more than 50%) with poor collateral cerebral circulation, or diminished cerebral perfusion on single photon emission computed tomography before and after administration of the intravenous dosage of acetazolamide.
RESULTS
The median age of patients was 53 years (range 44-79). The technical success rate was 100%. Mean pretreatment stenosis degree was 83.63 +/- 9.53% and 29.1 +/- 15.4% before and after angioplasty, respectively. Procedural-related complications occurred in four of 11 patients (36%), but none of the patients had permanent neurological deficit. All patients were available for an average follow-up period of 19.4 +/- 5.1 months. One patient had a stroke in the territory of angioplasty at two months after angioplasty. The stroke free survival rate at 30 days and 12 months was 100% and 91%, respectively. Restenosis over 50% was observed in three of 11 patients (27%); all were asymptomatic.
CONCLUSION
Intracranial angioplasty for symptomatic MCA stenosis refractory to medical therapy can be a treatment option to reduce the risk of further TIA or stroke.
MeSH Terms
Figure
Reference
-
1. Abou-Chebl A, Bashir Q, Yadav JS. Drug-eluting stents for the treatment of intracranial atherosclerosis: initial experience and midterm angiographic follow-up. Stroke. 2005; 12. 36(12):e165–e168. PMID: 16282539.
Article2. Bose A, Hartmann M, Henkes H, Liu HM, Teng MM, Szikora I, et al. A novel, self-expanding, nitinol stent in medically refractory intracranial atherosclerotic stenoses: the Wingspan study. Stroke. 2007; 5. 38(5):1531–1537. PMID: 17395864.3. Carlsson J, von Wagenheim B, Linder R, Anwari TM, Qvist J, Petersson I, et al. Is late stent thrombosis in drug-eluting stents a real clinical issue? A single-center experience and review of the literature. Clin Res Cardiol. 2007; 2. 96(2):86–93. PMID: 17180577.4. Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, et al. Warfarin-aspirin symptomatic intracranial disease trial investigators. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005; 3. 352(13):1305–1316. PMID: 15800226.
Article5. Connors JJ 3rd, Wojak JC. Percutaneous transluminal angioplasty for intracranial atherosclerotic lesions: evolution of technique and short-term results. J Neurosurg. 1999; 9. 91(3):415–423. PMID: 10470816.
Article6. Coward LJ, McCabe DJ, Ederle J, Featherstone RL, Clifton A, Brown MM. CAVATAS Investigators. Long-term outcome after angioplasty and stenting for symptomatic vertebral artery stenosis compared with medical treatment in the Carotid And Vertebral Artery Transluminal Angioplasty Study (CAVATAS): a randomized trial. Stroke. 2007; 5. 38(5):1526–1530. PMID: 17395869.7. Derdeyn CP, Chimowitz MI. Angioplasty and stenting for atherosclerotic intracranial stenosis: rationale for a randomized clinical trial. Neuroimaging Clin N Am. 2007; 8. 17(3):355–363. PMID: 17826637.
Article8. Fiorella D, Chow MM, Anderson M, Woo H, Rasmussen PA, Masaryk TJ. A 7-year experience with balloon-mounted coronary stents for the treatment of symptomatic vertebrobasilar intracranial atheromatous disease. Neurosurgery. 2007; 8. 61(2):236–242. discussion 242-3. PMID: 17762735.
Article9. Fiorella D, Levy EI, Turk AS, Albuquerque FC, Niemann DB, Aagaard-Kienitz B, et al. US multicenter experience with the wingspan stent system for the treatment of intracranial atheromatous disease: periprocedural results. Stroke. 2007; 3. 38(3):881–887. PMID: 17290030.
Article10. Gress DR, Smith WS, Dowd CF, Van Halbach V, Finley RJ, Higashida RT. Angioplasty for intracranial symptomatic vertebrobasilar ischemia. Neurosurgery. 2002; 7. 51(1):23–27. discussion 27-9. PMID: 12182423.
Article11. Kim JK, Ahn JY, Lee BH, Chung YS, Chung SS, Kim OJ, et al. Elective stenting for symptomatic middle cerebral artery stenosis presenting as transient ischaemic deficits or stroke attacks: short term arteriographical and clinical outcome. J Neurol Neurosurg Psychiatry. 2004; 6. 75(6):847–851. PMID: 15145998.
Article12. Lee CY, Yim MB. Primary stent therapy for symptomatic intracranial atherosclerotic stenosis: 1-year follow-up angiographic and midterm clinical outcomes. J Neurosurg. 2006; 8. 105(2):235–241.
Article13. Lee JH, Kwon SU, Lee JH, Suh DC, Kim JS. Percutaneous transluminal angioplasty for symptomatic middle cerebral artery stenosis: long-term follow-up. Cerebrovasc Dis. 2003; 15(1-2):90–97. PMID: 12499717.
Article14. Lutsep HL. Symptomatic intracranial stenosis: best medical treatment vs. intracranial stenting. Curr Opin Neurol. 2009; 2. 22(1):69–74. PMID: 19155764.
Article15. Marks MP, Marcellus ML, Do HM, Schraedley-Desmond PK, Steinberg GK, Tong DC, et al. Intracranial angioplasty without stenting for symptomatic atherosclerotic stenosis: long-term follow-up. AJNR Am J Neuroradiol. 2005; 3. 26(3):525–530. PMID: 15760860.16. Marks MP, Wojak JC, Al-Ali F, Jayaraman M, Marcellus ML, Connors JJ, et al. Angioplasty for symptomatic intracranial stenosis: clinical outcome. Stroke. 2006; 4. 37(4):1016–1020. PMID: 16497979.17. Miao ZR, Feng L, Li S, Zhu F, Ji X, Jiao L, et al. Treatment of symptomatic middle cerebral artery stenosis with balloon-mounted stents: long-term follow-up at a single center. Neurosurgery. 2009; 1. 64(1):79–84. discussion 84-5. PMID: 19050657.18. Mori T, Fukuoka M, Kazita K, Mori K. Follow-up study after intracranial percutaneous transluminal cerebral balloon angioplasty. AJNR Am J Neuroradiol. 1998; 9. 19(8):1525–1533. PMID: 9763389.
Article19. Puetz V, Gahn G, Becker U, Mucha D, Mueller A, Weir NU, et al. Endovascular therapy of symptomatic intracranial stenosis in patients with impaired regional cerebral blood flow or failure of medical therapy. AJNR Am J Neuroradiol. 2008; 2. 29(2):273–280. PMID: 17989370.
Article20. Qureshi AI, Hussein HM, El-Gengaihy A, Abdelmoula M, K Suri MF. Concurrent comparison of outcomes of primary angioplasty and of stent placement in high-risk patients with symptomatic intracranial stenosis. Neurosurgery. 2008; 5. 62(5):1053–1060. discussion 1060-2. PMID: 18580803.
Article21. Suh DC, Kim JK, Choi JW, Choi BS, Pyun HW, Choi YJ, et al. Intracranial stenting of severe symptomatic intracranial stenosis: results of 100 consecutive patients. AJNR Am J Neuroradiol. 2008; 4. 29(4):781–785. PMID: 18310234.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Stenting of Symptomatic Middle Cerebral Artery Stenosis: Case Report
- Guideline for Intracranial Stenting of Symptomatic Intracranial Artery Stenosis: Preliminary Report
- Stent-Assisted Angioplasty for Symptomatic Middle Cerebral Artery Stenosis: Short-term Arteriographic and Clinical Outcome
- Improved Cerebral Perfusion after Stent-Assisted Angioplasty for Middle Cerebral Artery Stenosis
- Clinical Outcome of Balloon Angioplasty for Symptomatic Middle Cerebral Artery Stenosis