Anat Cell Biol.
2014 Sep;47(3):162-170. 10.5115/acb.2014.47.3.162.
Effect of nicotine on the structure of cochlea of guinea pigs
- Affiliations
-
- 1Department of Histology, Faculty of Medicine, Assiut University, Assiut, Egypt. selgar1@hotmail.com
- KMID: 1882591
- DOI: http://doi.org/10.5115/acb.2014.47.3.162
Abstract
- Smoking has been positively associated with hearing loss in human. However, its effect on the cochlea has not been previously evaluated. Aim of work is to investigate the effect of nicotine, which is the primary pharmacological component of tobacco, on the structure of the cochlea of adult male guinea pigs. Fifteen male guinea pigs were classified into two groups: group I (control) and group II (nicotine treated group). Group II was further subdivided into two subgroups; IIA and IIB according to the dose of nicotine (3 mg/kg and 6 mg/kg, respectively). The cochlea was harvested and processed for light microscopy, transmission electron microscopy and scanning electron microscopy. Nicotine administration induced damage of outer hair cells which were distorted in shape with vacuolated cytoplasm and heterochromatic nuclei. Topography revealed damage of the stereocilia which included disorganization, bent and limp or complete loss and expansion of the surrounding supporting cells. These changes were more pronounced in the basal turn of the cochlea and mainly involved the outer hair cells. High dose induced more damage and resulted in protrusion of the apical poles of hair cells (blebing), particularly the outer two rows. Nicotine is proved to be harmful to the cells of the cochlea, particularly the outer hair cells of the basal turn. High doses induce blebing of hair cells.
Keyword
MeSH Terms
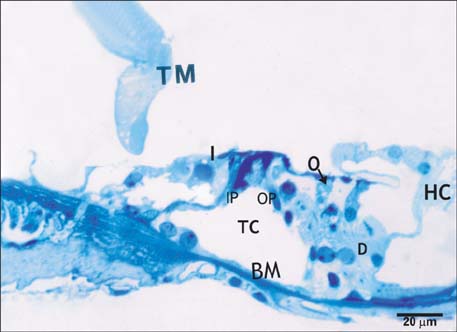
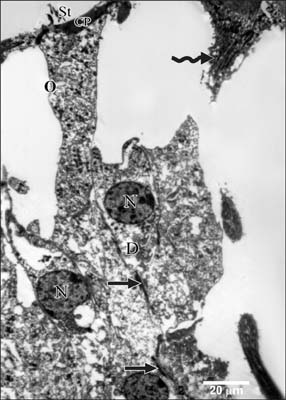
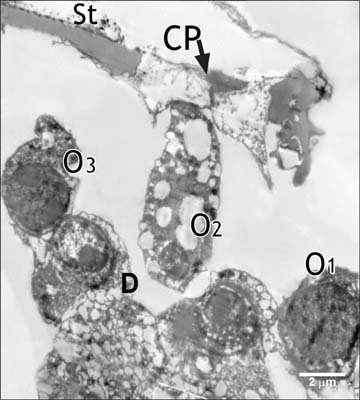
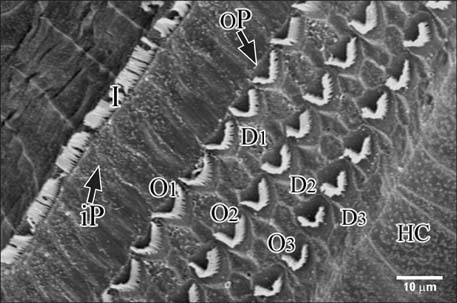
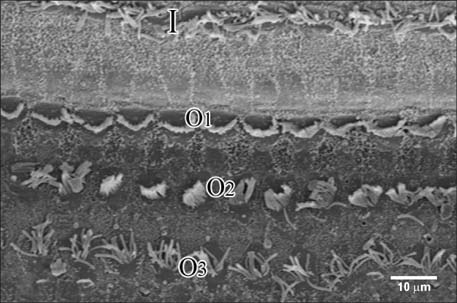
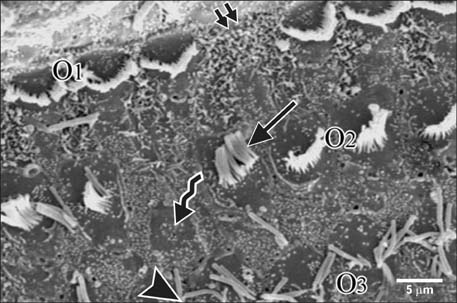
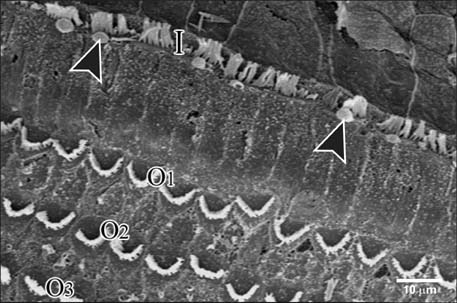
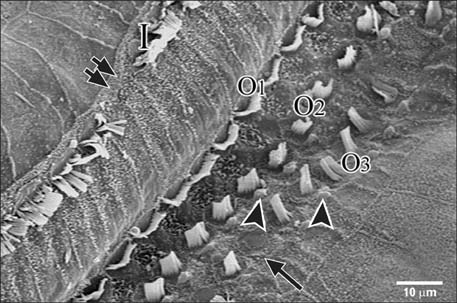
Figure
Cited by 1 articles
-
Impact of Nicotine Exposure on Hair Cell Toxicity and Embryotoxicity During Zebrafish Development
Myung Hoon Yoo, Yoon Chan Rah, Saemi Park, Soonil Koun, Gi Jung Im, Sung Won Chae, Hak Hyun Jung, June Choi
Clin Exp Otorhinolaryngol. 2018;11(2):109-117. doi: 10.21053/ceo.2017.00857.
Reference
-
1. Cunningham CS, Javors MA, McMahon LR. Pharmacologic characterization of a nicotine-discriminative stimulus in rhesus monkeys. J Pharmacol Exp Ther. 2012; 341:840–849.2. Hiremagalur B, Sabban EL. Nicotine elicits changes in expression of adrenal catecholamine biosynthetic enzymes, neuropeptide Y and immediate early genes by injection but not continuous administration. Brain Res Mol Brain Res. 1995; 32:109–115.3. Ingram JR, Routledge P, Rhodes J, Marshall RW, Buss DC, Evans BK, Feyerabend C, Thomas GA. Nicotine enemas for treatment of ulcerative colitis: a study of the pharmacokinetics and adverse events associated with three doses of nicotine. Aliment Pharmacol Ther. 2004; 20:859–865.4. McClernon FJ, Hiott FB, Westman EC, Rose JE, Levin ED. Transdermal nicotine attenuates depression symptoms in nonsmokers: a double-blind, placebo-controlled trial. Psychopharmacology (Berl). 2006; 189:125–133.5. Quik M, Perez XA, Bordia T. Nicotine as a potential neuroprotective agent for Parkinson's disease. Mov Disord. 2012; 27:947–957.6. Paschoal CP, Azevedo MF. Cigarette smoking as a risk factor for auditory problems. Braz J Otorhinolaryngol. 2009; 75:893–902.7. Vinay . Effect of smoking on transient evoked otoacoustic emissions and contralateral suppression. Auris Nasus Larynx. 2010; 37:299–302.8. Slepecky NB. Structure of the mammalian cochlea. In : Dallos P, Popper AN, Fay RR, editors. The Cochlea. New York: Springer;1996. p. 44–129.9. Daudet N, Vago P, Ripoll C, Humbert G, Pujol R, Lenoir M. Characterization of atypical cells in the juvenile rat organ of corti after aminoglycoside ototoxicity. J Comp Neurol. 1998; 401:145–162.10. Santi PA, Tsuprun VL. Cochlear microanatomy and ultrastructure. In : Jahn AF, Santos-Sacchi J, editors. Physiology of the Ear. San Diego: Singular Publishing;2001. p. 257–281.11. Hyppolito MA, de Oliveira AA, Lessa RM, Rossato M. [Amifostine otoprotection to cisplatin ototoxicity: a guinea pig study using otoacoustic emission distortion products (DPOEA) and scanning electron microscopy]. Braz J Otorhinolaryngol. 2005; 71:268–273.12. Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM. Guidelines on nicotine dose selection for in vivo research. Psychopharmacology (Berl). 2007; 190:269–319.13. Gupta PD. Ultrastructural study on semithin section. Sci Tools. 1983; 30:6–7.14. Reynolds ES. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963; 17:208–212.15. Albuquerque AA, Rossato M, Oliveira JA, Hyppolito MA. Understanding the anatomy of ears from guinea pigs and rats and its use in basic otologic research. Braz J Otorhinolaryngol. 2009; 75:43–49.16. Berger F, Gage FH, Vijayaraghavan S. Nicotinic receptor-induced apoptotic cell death of hippocampal progenitor cells. J Neurosci. 1998; 18:6871–6881.17. Matsunobu T, Chung JW, Schacht J. Acetylcholine-evoked calcium increases in Deiters' cells of the guinea pig cochlea suggest alpha9-like receptors. J Neurosci Res. 2001; 63:252–256.18. Dodson HC, Bannister LH, Douek EE. The effects of combined gentamicin and white noise on the spiral organ of young guinea pigs. A structural study. Acta Otolaryngol. 1982; 94:193–202.19. Forge A. Outer hair cell loss and supporting cell expansion following chronic gentamicin treatment. Hear Res. 1985; 19:171–182.20. Taylor RR, Jagger DJ, Forge A. Defining the cellular environment in the organ of Corti following extensive hair cell loss: a basis for future sensory cell replacement in the Cochlea. PLoS One. 2012; 7:e30577.21. Pickles JO, Comis SD, Osborne MP. Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res. 1984; 15:103–112.22. Comis SD, Pickles JO, Osborne MP. Osmium tetroxide postfixation in relation to the crosslinkage and spatial organization of stereocilia in the guinea-pig cochlea. J Neurocytol. 1985; 14:113–130.23. Ricci AJ, Kachar B, Gale J, Van Netten SM. Mechano-electrical transduction: new insights into old ideas. J Membr Biol. 2006; 209:71–88.24. Horner KC, Cazals Y, Guieu R, Lenoir M, Sauze N. Experimental estrogen-induced hyperprolactinemia results in bone-related hearing loss in the guinea pig. Am J Physiol Endocrinol Metab. 2007; 293:E1224–E1232.25. Durante AS, Pucci B, Gudayol N, Massa B, Gameiro M, Lopes C. Tobacco smoke exposure during childhood: effect on cochlear physiology. Int J Environ Res Public Health. 2013; 10:5257–5265.26. Harpur ES, Bridges JB. An evaluation of the use of scanning and transmission electronmicroscopy in a study of the gentamicin-damaged guinea-pig organ of Corti. J Laryngol Otol. 1979; 93:7–23.27. Brownell WE, Bader CR, Bertrand D, de Ribaupierre Y. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 1985; 227:194–196.28. Bohne BA, Clark WW. Growth of hearing loss and cochlear lesion with increasing duration of noise exposure. In : Harmernik RP, Handerson D, Salvi R, editors. New Perspective on Noise-Induced Hearing Loss. New York: Raven Press;1987. p. 208–302.29. Hazell JW, Jastreboff PJ. Tinnitus. I: Auditory mechanisms: a model for tinnitus and hearing impairment. J Otolaryngol. 1990; 19:1–5.30. Lenoir M, Puel JL. Dose-dependent changes in the rat cochlea following aminoglycoside intoxication. II. Histological study. Hear Res. 1987; 26:199–209.31. Ramírez-Camacho R, García-Berrocal JR, Trinidad A, Verdaguer JM, Nevado J. Blebs in inner and outer hair cells: a pathophysiological hypothesis. J Laryngol Otol. 2008; 122:1151–1155.32. Zeddies DG, Siegel JH. A biophysical model of an inner hair cell. J Acoust Soc Am. 2004; 116:426–441.33. Shi X, Gillespie PG, Nuttall AL. Na+ influx triggers bleb formation on inner hair cells. Am J Physiol Cell Physiol. 2005; 288:C1332–C1341.34. Coleman ML, Sahai EA, Yeo M, Bosch M, Dewar A, Olson MF. Membrane blebbing during apoptosis results from caspase-mediated activation of ROCK I. Nat Cell Biol. 2001; 3:339–345.35. Raphael Y, Altschuler RA. Structure and innervation of the cochlea. Brain Res Bull. 2003; 60:397–422.36. Nakanishi N, Okamoto M, Nakamura K, Suzuki K, Tatara K. Cigarette smoking and risk for hearing impairment: a longitudinal study in Japanese male office workers. J Occup Environ Med. 2000; 42:1045–1049.37. Wild DC, Brewster MJ, Banerjee AR. Noise-induced hearing loss is exacerbated by long-term smoking. Clin Otolaryngol. 2005; 30:517–520.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effects of Ginkgo biloba extract on the cochlear damage induced by local gentamicin installation in guinea pigs
- Histological study on the effect of nicotine on adult male guinea pig thin skin
- Preventive Effect of Fosfomycin on Cisplatin Induced Ototoxicity in Cochlea of Guinea Pig
- Experimental Dermatitis by Pityrosporum ovale in Guinea Pigs
- Auditory Effects of Microperfused Lidocaine on Guinea Pig Cochlea