Korean Circ J.
2010 Nov;40(11):565-572. 10.4070/kcj.2010.40.11.565.
Examining the Relationship Between Triggering Activities and the Circadian Distribution of Acute Aortic Dissection
- Affiliations
-
- 1Department of Internal Medicine, Kyungpook National University Hospital, Daegu, Korea. choyk@mail.knu.ac.kr
- 2Department of Internal Medicine, CHA University, CHA Gumi Medical Center, Gumi, Korea.
- 3Department of Internal Medicine, Busan St. Mary's Medical Center, Busan, Korea.
- KMID: 1826175
- DOI: http://doi.org/10.4070/kcj.2010.40.11.565
Abstract
- BACKGROUND AND OBJECTIVES
There are limited data examining triggering activities and circadian distribution at the onset of acute aortic dissection (AAD) in the context of diagnostic and anatomical classification. The aim of this study was to further investigate this relationship between triggering activities and circadian distribution at the onset of AAD according to diagnostic and anatomic classification.
SUBJECTS AND METHODS
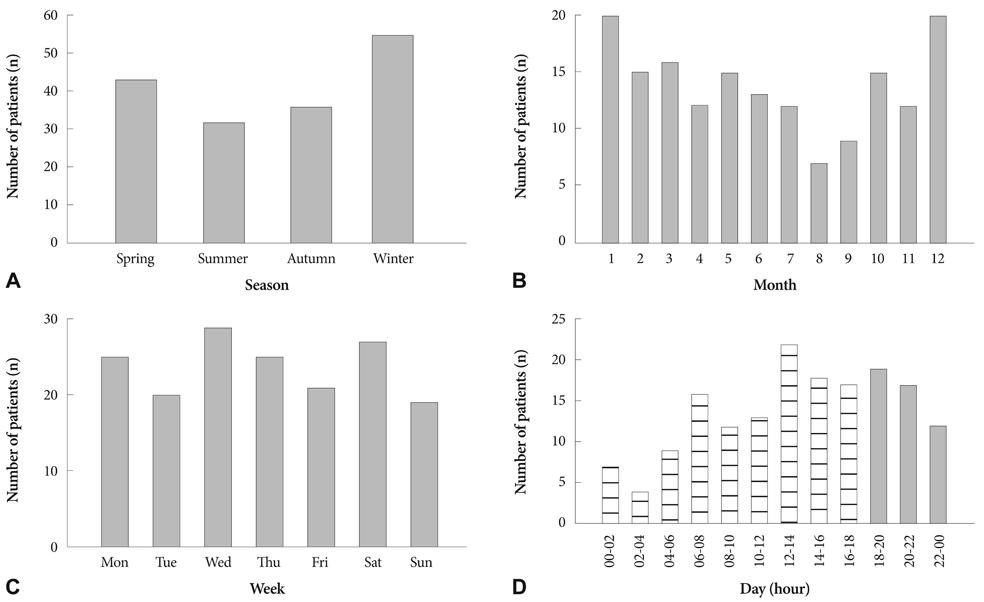
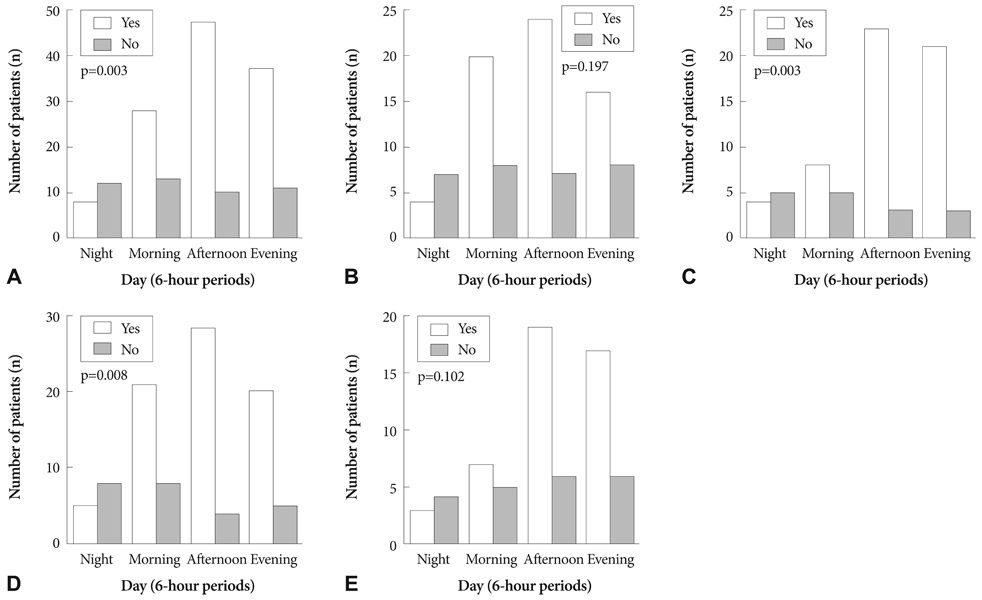
A total of 166 patients with AAD admitted to Kyungpook National University Hospital between July 2001 and June 2009 were included. To assess the influence of diagnostic and anatomical classification, we categorized the patients into intramural hematoma (IMH) group (n=67)/non-IMH group (n=99) and Stanford type A (AAD-A, n=94)/type B (AAD-B, n=72). To evaluate circadian distribution, the day was divided into four 6-hour periods: night (00-06 hours), morning (06-12 hours), afternoon (12-18 hours), and evening (18-00 hours).
RESULTS
Most (72%) AAD episodes were related to physical (53%) and mental activities (19%), with about one-third occurring during the afternoon, and only 12% occurring at night. No differences in triggering activities or circadian distribution were observed among the groups. Waking hours including morning, afternoon, and evening correlated with triggering activities (p=0.003). These relationships were observed for the non-IMH (p=0.008) and AAD-B (p=0.003) cases. The remaining categories had similar relationships, but did not reach statistical significance.
CONCLUSION
Our findings suggest differences in the relationship between triggering activities and the circadian distribution of the onset of AAD according to diagnostic and anatomical classification.
Keyword
MeSH Terms
Figure
Reference
-
1. Mittleman MA, Maclure M, Tofler GH, Serwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. N Engl J Med. 1993. 329:1677–1683.2. Willich SN, Lewis M, Löwel H, Arntz HR, Schubert F, Schröder R. Physical exertion as a trigger of acute myocardial infarction. N Engl J Med. 1993. 329:1684–1690.3. Krantz DS, Kop WJ, Gabbay FH, et al. Circadian variation of ambulatory myocardial ischemia: triggering by daily activities and evidence for an endogenous circadian component. Circulation. 1996. 93:1364–1371.4. Kono T, Morita H, Nishina T, et al. Circadian variations of onset of acute myocardial infarction and efficacy of thrombolytic therapy. J Am Coll Cardiol. 1996. 27:774–778.5. Marchant B, Ranjadayalan K, Stevenson R, Wilkinson P, Timmis AD. Circadian and seasonal factors in the pathogenesis of acute myocardial infarction: the influence of environmental temperature. Br Heart J. 1993. 69:385–387.6. Gnecchi-Ruscone T, Piccaluga E, Guzzetti S, Contini M, Montano N, Nicolis E. Morning and Monday: critical periods for the onset of acute myocardial infarction. The GISSI 2 Study experience. Eur Heart J. 1994. 15:882–887.7. Willich SN, Levy D, Rocco MB, Tofler GH, Stone PH, Muller JE. Circadian variation in the incidence of sudden cardiac death in the Framingham Heart Study population. Am J Cardiol. 1987. 60:801–806.8. Gallerani M, Portaluppi F, Maida G, et al. Circadian and circannual rhythmicity in the occurrence of subarachnoid hemorrhage. Stroke. 1996. 27:1793–1797.9. Kojima S, Sumiyoshi M, Nakata Y, Daida H. Triggers and circadian distribution of the onset of acute aortic dissection. Circ J. 2002. 66:232–235.10. Mehta RH, Manfredini R, Hassan F, et al. Chronobiological patterns of acute aortic dissection. Circulation. 2002. 106:1110–1115.11. Kim JK, Park SW, Jeong JO, et al. Clinical features and prognosis of acute aortic intramural hemorrhage compared with those of acute aortic dissection: a single center experience. Jpn Heart J. 2001. 42:91–100.12. Hwang GS, Kim YH, Lee HS, et al. Clinical comparison of aortic intramural hemorrhage with aortic dissection involving the ascending aorta. Korean Circ J. 2000. 30:440–447.13. Song JK, Kim HS, Song JM, et al. Multicenter longitudinal follow-up clinical study comparing the natural course of medically-treated patients with aortic dissection and aortic intramural hematoma. Korean Circ J. 2001. 31:592–601.14. Lee IS, Kang DH, Song JK, et al. Clinical and echocardiographic outcome of aortic intramural hemorrhage compared with acute aortic dissection. Korean Circ J. 1998. 28:749–756.15. Mohr-Kahaly S, Erbel R, Kearney P, Puth M, Meyer J. Aortic intramural hemorrhage visualized by transesophageal echocardiography: findings and prognostic implications. J Am Coll Cardiol. 1994. 23:658–664.16. Nienaber CA, von Kodolitsch Y, Petersen B, et al. Intramural hemorrhage of the thoracic aorta: diagnostic and therapeutic implications. Circulation. 1995. 92:1465–1472.17. Harris KM, Braverman AC, Gutierrez FR, Barzilai B, Dávila-Román VG. Transesophageal echocardiographic and clinical features of aortic intramural hematoma. J Thorac Cardiovasc Surg. 1997. 114:619–626.18. Sueyoshi E, Matsuoka Y, Sakamoto I, Uetani M, Hayashi K, Narimatsu M. Fate of intramural hematoma of the aorta: CT evaluation. J Comput Assist Tomogr. 1997. 21:931–938.19. Daily PO, Trueblood HW, Stinson EB, Wuerflein RD, Shumway NE. Management of acute aortic dissections. Ann Thorac Surg. 1970. 10:237–247.20. Matsuo H. The thrombosed type of aortic dissection: its clinical features and diagnosis. Int J Angiol. 1998. 7:329–334.21. Turjanmaa V, Tuomisto M, Fredrikson M, Kalli S, Uusitalo A. Blood pressure and heart rate variability and reactivity as related to daily activities in normotensive men measured with 24-h intra-arterial recording. J Hypertens. 1991. 9:665–673.22. Fogelholm RR, Turjanmaa VM, Nuutila MT, Murros KE, Sarna S. Diurnal blood pressure variations and onset of subarachnoid haemorrhage: a population-based study. J Hypertens. 1995. 13:495–498.23. Sumiyoshi M, Kojima S, Arima M, et al. Circadian, weekly, and seasonal variation at the onset of acute aortic dissection. Am J Cardiol. 2002. 89:619–623.24. Izzo JL Jr, Larrabee PS, Sander E, Lillis LM. Hemodynamics of seasonal adaptation. Am J Hypertens. 1990. 3:405–407.25. Nienaber CA, Fattori R, Mehta RH, et al. Gender-related differences in acute aortic dissection. Circulation. 2004. 109:3014–3021.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Placement of Endovascular Stent Graft in Acute Malperfusion Syndrome After Acute Type II Aortic Dissection
- Acute Type II Aortic Dissection with Severe Aortic Regurgitation and Chronic Descending Aortic Dissection in Pregnant Patient with Marfan Syndrome
- A Case of Total Aortic Arch Replacement with Root Plasty with Right Coronary Artery Bypass and Distal Open Stent-graft Insertion in Acute Type I Aortic Dissection
- A Case of Acute Aortic Dissection with Dynamic ST Changes in Electrocardiogram
- Retrograde Aortic Dissection after Thoracic Endovascular Aortic Repair for Descending Aorta: 2 case reports