J Korean Soc Transplant.
2009 Dec;23(3):203-213. 10.4285/jkstn.2009.23.3.203.
Current Status and Future Perspectives of Xenotransplantation
- Affiliations
-
- 1Department of Microbiology and Immunology, Seoul National University College of Medicine, Seoul, Korea. chgpark@snu.ac.kr
- 2Xenotransplantation Research Center, Seoul National University College of Medicine, Seoul, Korea.
- 3Transplantation Research Institute SNUMRC, Seoul National University College of Medicine, Seoul, Korea.
- 4Cancer Research Institute and TIMRC, Seoul National University College of Medicine, Seoul, Korea.
- 5Department of Surgery, Seoul National University College of Medicine, Seoul, Korea.
- KMID: 1819842
- DOI: http://doi.org/10.4285/jkstn.2009.23.3.203
Abstract
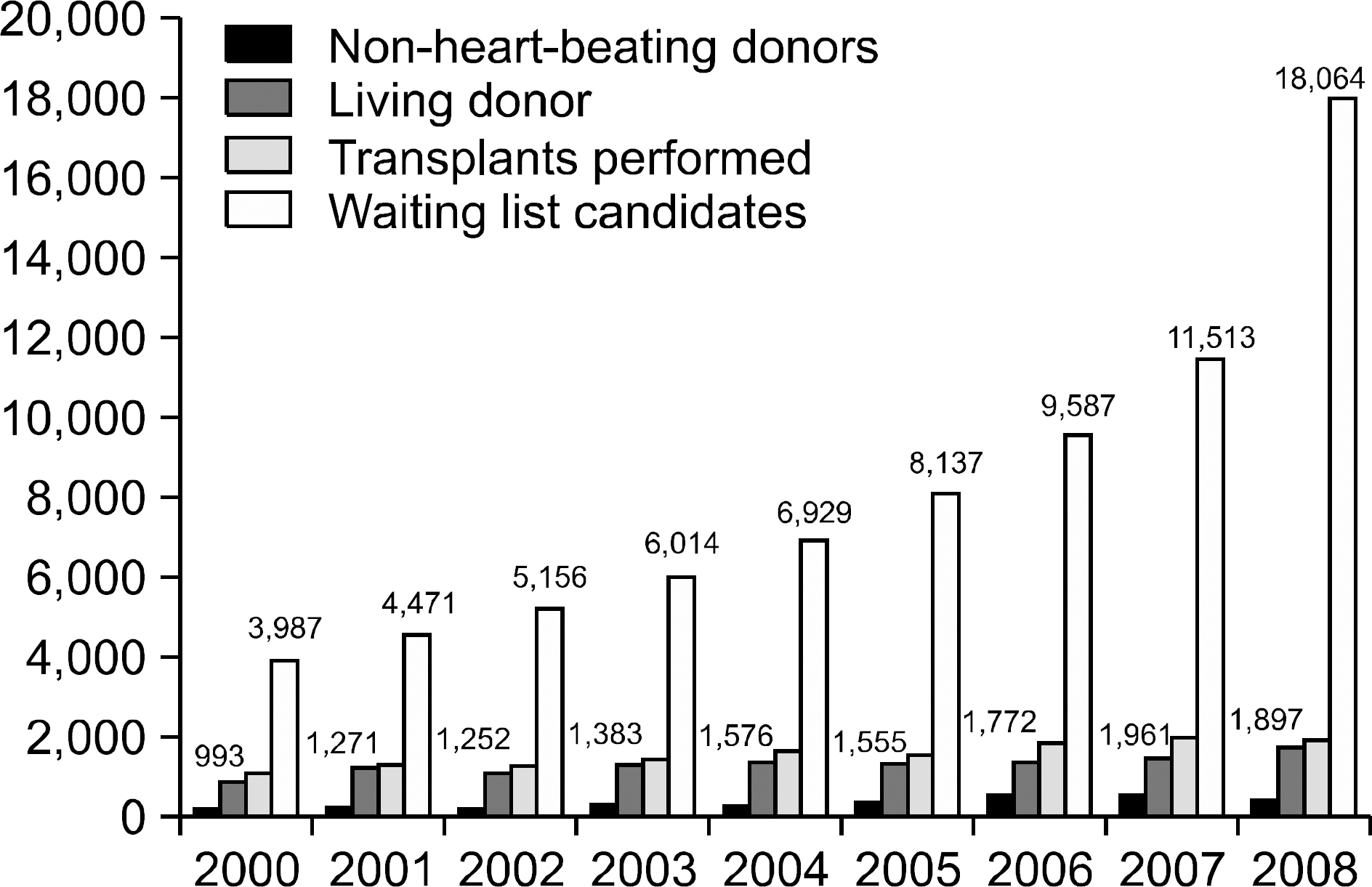
- Xenotransplantation using pigs as the transplant source holds great promise to resolve the severe shortage of human organ donors. Although stem-cell-derived organ and tissue regeneration have a potential to solve this as well for the future, it still remains as very early experimental phase. Likewise, artificial organs and mechanical devices have been simply used for bridge therapy to transplant. Therefore, xenotransplantation might provide the most imminent solution to the scarcity of human organ donors. In the last two decades, major progress has been made in understanding the mechanisms of xenografts rejection, zoonotic infections including porcine endogenous retrovirus (PERV) and production of genetically engineered pigs including alpha1,3-galactosyltransferase-deficient pigs. With these elaborations, it is now on the threshold of first clinical application. Particularly promising first target is porcine pancreatic islet xenotransplantation. Graft survival has been prolonged to almost one year in the non-human primate study and is waiting for the development of relatively non-toxic or clinically applicable immunosuppressive or tolerance-inducing regimens. This review highlights the currently known obstacles to translate xenotransplantation into clinical therapies and the possible strategies to overcome these hurdles, as well as current status and future perspective for clinical xenotransplantaion.
MeSH Terms
Figure
Cited by 1 articles
-
Safety for Expanding Living-Donor Criteria in Renal Transplantation
Hyeon Seok Hwang, Suk Young Kim
J Korean Soc Transplant. 2010;24(2):80-86. doi: 10.4285/jkstn.2010.24.2.80.
Reference
-
1). Groth CG, Korsgren O, Tibell A, Tollemar J, Möller E, Bolinder J, et al. Transplantation of porcine fetal pancreas to diabetic patients. Lancet. 1994; 344:1402–4.
Article2). Rood PP, Cooper DK. Islet xenotransplantation: are we really ready for clinical trials? Am J Transplant. 2006; 6:1269–74.
Article3). Hering BJ, Cooper DK, Cozzi E, Schuurman HJ, Korbutt GS, Denner J, et al. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes– executive summary. Xenotransplantation. 2009; 16:196–202.4). Galili U. Interaction of the natural anti-Gal antibody with alpha-galactosyl epitopes: a major obstacle for xenotransplantation in humans. Immunol Today. 1993; 14:480–2.5). Schuurman HJ, Cheng J, Lam T. Pathology of xenograft rejection: a commentary. Xenotransplantation. 2003; 10:293–9.
Article6). Schuurman HJ, Pino-Chavez G, Phillips MJ, Thomas L, White DJ, Cozzi E. Incidence of hyperacute rejection in pig-to-primate transplantation using organs from hDAF- transgenic donors. Transplantation. 2002; 73:1146–51.7). Cozzi E, Bosio E, Seveso M, Vadori M, Ancona E. Xenotransplantation-current status and future perspectives. Br Med Bull. 2005; 75-76:99–114.
Article8). Cowan PJ. Coagulation and the xenograft endothelium. Xenotransplantation. 2007; 14:7–12.
Article9). Diamond LE, Quinn CM, Martin MJ, Lawson J, Platt JL, Logan JS. A human CD46 transgenic pig model system for the study of discordant xenotransplantation. Transplantation. 2001; 71:132–42.
Article10). Niemann H, Verhoeyen E, Wonigeit K, Lorenz R, Hecker J, Schwinzer R, et al. Cytomegalovirus early promoter induced expression of hCD59 in porcine organs provides protection against hyperacute rejection. Transplantation. 2001; 72:1898–906.11). Lai L, Kolber-Simonds D, Park KW, Cheong HT, Greenstein JL, Im GS, et al. Production of alpha-1,3-gal-actosyltransferase knockout pigs by nuclear transfer cloning. Science. 2002; 295:1089–92.12). Kuwaki K, Tseng YL, Dor FJ, Shimizu A, Houser SL, Sanderson TM, et al. Heart transplantation in baboons using alpha1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat Med. 2005; 11:29–31.13). Milland J, Christiansen D, Lazarus BD, Taylor SG, Xing PX, Sandrin MS. The molecular basis for galalpha(1,3)gal expression in animals with a deletion of the alpha1,3gal-actosyltransferase gene. J Immunol. 2006; 176:2448–54.14). Cascalho M, Platt JL. The immunological barrier to xenotransplantation. Immunity. 2001; 14:437–46.
Article15). Ramírez P, Montoya MJ, Ríos A, García Palenciano C, Majado M, Chávez R, et al. Prevention of hyperacute rejection in a model of orthotopic liver xenotransplantation from pig to baboon using polytransgenic pig livers (CD55, CD59, and H-transferase). Transplant Proc. 2005; 37:4103–6.
Article16). Cozzi E, Bhatti F, Schmoeckel M, Chavez G, Smith KG, Zaidi A, et al. Long-term survival of nonhuman primates receiving life-supporting transgenic porcine kidney xenografts. Transplantation. 2000; 70:15–21.17). Chen G, Qian H, Starzl T, Sun H, Garcia B, Wang X, et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat Med. 2005; 11:1295–8.
Article18). Davila E, Byrne GW, LaBreche PT, McGregor HC, Schwab AK, Davies WR, et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation. 2006; 13:31–40.
Article19). Yang YG, Sykes M. Xenotransplantation: current status and a perspective on the future. Nat Rev Immunol. 2007; 7:519–31.
Article20). Simeonovic CJ. Xenogeneic islet transplantation. Xenotransplantation. 1999; 6:1–5.
Article21). Cardona K, Korbutt GS, Milas Z, Lyon J, Cano J, Jiang W, et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat Med. 2006; 12:304–6.
Article22). Hering BJ, Wijkstrom M, Graham ML, Hårdstedt M, Aasheim TC, Jie T, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006; 12:301–3.
Article23). Tseng YL, Dor FJ, Kuwaki K, Ryan D, Wood J, Denaro M, et al. Bone marrow transplantation from alpha1,3-ga-lactosyltransferase gene-knockout pigs in baboons. Xenotransplantation. 2004; 11:361–70.
Article24). Cosimi AB, Sachs DH. Mixed chimerism and transplantation tolerance. Transplantation. 2004; 77:943–6.
Article25). Yamada K, Yazawa K, Shimizu A, Iwanaga T, Hisashi Y, Nuhn M, et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of alpha1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat Med. 2005; 11:32–4.26). Itescu S, Kwiatkowski P, Artrip JH, Wang SF, Ankersmit J, Minanov OP, et al. Role of natural killer cells, macrophages, and accessory molecule interactions in the rejection of pig-to-primate xenografts beyond the hyperacute period. Hum Immunol. 1998; 59:275–86.
Article27). Candinas D, Belliveau S, Koyamada N, Miyatake T, Hechenleitner P, Mark W, et al. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation. 1996; 62:1920–7.28). Sprangers B, Waer M, Billiau AD. Xenotransplantation: where are we in 2008? Kidney Int. 2008; 74:14–21.
Article29). Lilienfeld BG, Crew MD, Forte P, Baumann BC, Seebach JD. Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation. 2007; 14:126–34.
Article30). Lin Y, Vandeputte M, Waer M. Contribution of activated macrophages to the process of delayed xenograft rejection. Transplantation. 1997; 64:1677–83.31). Wallgren AC, Karlsson-Parra A, Korsgren O. The main infiltrating cell in xenograft rejection is a CD4+ macrophage and not a T lymphocyte. Transplantation. 1995; 60:594–601.
Article32). Oldenborg PA, Zheleznyak A, Fang YF, Lagenaur CF, Gresham HD, Lindberg FP. Role of CD47 as a marker of self on red blood cells. Science. 2000; 288:2051–4.
Article33). Ide K, Wang H, Tahara H, Liu J, Wang X, Asahara T, et al. Role for CD47-SIRPalpha signaling in xenograft rejection by macrophages. Proc Natl Acad Sci U S A. 2007; 104:5062–6.34). Baumann BC, Stussi G, Huggel K, Rieben R, Seebach JD. Reactivity of human natural antibodies to endothelial cells from Galalpha(1,3)Gal-deficient pigs. Transplantation. 2007; 83:193–201.35). Morozumi K, Kobayashi T, Usami T, Oikawa T, Ohtsuka Y, Kato M, et al. Significance of histochemical expression of Hanganutziu-Deicher antigens in pig, baboon and human tissues. Transplant Proc. 1999; 31:942–4.
Article36). Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, et al. Generation of C5a in the absence of C3: a new complement activation pathway. Nat Med. 2006; 12:682–7.
Article37). Selander B, Mårtensson U, Weintraub A, Holmström E, Matsushita M, Thiel S, et al. Mannan-binding lectin acti-vates C3 and the alternative complement pathway with-out involvement of C2. J Clin Invest. 2006; 116:1425–34.
Article38). Khalpey Z, Yuen AH, Kalsi KK, Kochan Z, Karbowska J, Slominska EM, et al. Loss of ecto-5'nucleotidase from porcine endothelial cells after exposure to human blood: Implications for xenotransplantation. Biochim Biophys Acta. 2005; 1741:191–8.
Article39. Morrell CN, Sun H, Swaim AM, Baldwin WM 3rd. Platelets an inflammatory force in transplantation. Am J Transplant. 2007; 7:2447–54.
Article40). Wagner DD, Frenette PS. The vessel wall and its interactions. Blood. 2008; 111:5271–81.
Article41). Schulte Am Esch J 2nd, Robson SC, Knoefel WT, Hosch SB, Rogiers X. O-linked glycosylation and functional incompatibility of porcine von Willebrand factor for human platelet GPIb receptors. Xenotransplantation. 2005; 12:30–7.
Article42). Roussel JC, Moran CJ, Salvaris EJ, Nandurkar HH, d'Apice AJ, Cowan PJ. Pig thrombomodulin binds human thrombin but is a poor cofactor for activation of human protein C and TAFI. Am J Transplant. 2008; 8:1101–12.
Article43). Lin CC, Cooper DK, Dorling A. Coagulation dysregula-tion as a barrier to xenotransplantation in the primate. Transpl Immunol. 2009; 21:75–80.
Article44). Ezzelarab M, Cooper DK. The potential of statins in xenotransplantation. Xenotransplantation. 2007; 14:100–3.
Article45). Aikawa M, Rabkin E, Sugiyama S, Voglic SJ, Fukumoto Y, Furukawa Y, et al. An HMG-CoA reductase inhibitor, cerivastatin, suppresses growth of macrophages expressing matrix metalloproteinases and tissue factor in vivo and in vitro. Circulation. 2001; 103:276–83.
Article46). Ledoux S, Laouari D, Essig M, Runembert I, Trugnan G, Michel JB, et al. Lovastatin enhances ecto-5'-nucleoti-dase activity and cell surface expression in endothelial cells: implication of rho-family GTPases. Circ Res. 2002; 90:420–7.47). Wilson CA. Porcine endogenous retroviruses and xenotransplantation. Cell Mol Life Sci. 2008; 65:3399–412.48). Bartosch B, Stefanidis D, Myers R, Weiss R, Patience C, Takeuchi Y. Evidence and consequence of porcine endogenous retrovirus recombination. J Virol. 2004; 78:13880–90.
Article49). Scobie L, Takeuchi Y. Porcine endogenous retrovirus and other viruses in xenotransplantation. Curr Opin Organ Transplant. 2009; 14:175–9.
Article50). Denner J, Schuurman HJ, Patience C. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes–chapter 5: Strategies to pre-vent transmission of porcine endogenous retroviruses. Xenotransplantation. 2009; 16:239–48.51). Mueller NJ, Barth RN, Yamamoto S, Kitamura H, Patience C, Yamada K, et al. Activation of cytomegalovirus in pig-to-primate organ xenotransplantation. J Virol. 2002; 76:4734–40.
Article52). Mueller NJ, Livingston C, Knosalla C, Barth RN, Yamamoto S, Gollackner B, et al. Activation of porcine cytomegalovirus, but not porcine lymphotropic herpesvirus, in pig-to-baboon xenotransplantation. J Infect Dis. 2004; 189:1628–33.
Article53). Sykes M, d'Apice A, Sandrin M.IXA Ethics Committee. Position paper of the Ethics Committee of the International Xenotransplantation Association. Xenotransplantation. 2003; 10:194–203.
Article54). Dufrane D, Gianello P. Pig islets for clinical islet xenotransplantation. Curr Opin Nephrol Hypertens. 2009; 18:495–500.
Article55). McKenzie IF, Koulmanda M, Mandel TE, Sandrin MS. Pig islet xenografts are susceptible to "anti-pig" but not Gal alpha(1,3)Gal antibody plus complement in Gal o/o mice. J Immunol. 1998; 161:5116–9.56). van der Windt DJ, Bottino R, Casu A, Campanile N, Cooper DK. Rapid loss of intraportally transplanted islets: an overview of pathophysiology and preventive strategies. Xenotransplantation. 2007; 14:288–97.
Article57). Giovagnoli S, Luca G, Casaburi I, Blasi P, Macchiarulo G, Ricci M, et al. Long-term delivery of superoxide dis-mutase and catalase entrapped in poly(lactide-co-glycolide) microspheres: in vitro effects on isolated neonatal porcine pancreatic cell clusters. J Control Release. 2005; 107:65–77.58). van der Windt DJ, Bottino R, Casu A, Campanile N, Smetanka C, He J, et al. Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hcd46 transgenic porcine islets. Am J Transplant. 2009; 9:2716–26.
Article59). Cooper DK, Casu A. The International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes–chapter 4: Pre-clinical efficacy and complication data required to justify a clinical trial. Xenotransplantation. 2009; 16:229–38.60). Kim JH, Kim HI, Lee KW, Yu JE, Kim SH, Park HS, et al. Influence of strain and age differences on the yields of porcine islet isolation: extremely high islet yields from SPF CMS miniature pigs. Xenotransplantation. 2007; 14:60–6.
Article61). Kim HI, Lee SY, Jin SM, Kim KS, Yu JE, Yeom SC, et al. Parameters for successful pig islet isolation as determined using 68 specific-pathogen-free miniature pigs. Xenotransplantation. 2009; 16:11–8.
Article62). Sykes M. Commentary: World Health Assembly resolution 57.18 on xenotransplantation. Transplantation. 2005; 79:636–7.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Current Status of Solid Organ Xenotransplantation
- Current status of pancreatic islet xenotransplantation
- Current Status and Future Perspectives of Xenotransplantation and Stem Cell Research in Transplantation Field
- Overcoming Immunological Barriers in Xenotransplantation: A Historical Review of Previous Research and Future Directions
- The role of artificial intelligence in pancreatic surgery: Current and future perspectives