J Gynecol Oncol.
2014 Jan;25(1):51-57. 10.3802/jgo.2014.25.1.51.
A longitudinal analysis with CA-125 to predict overall survival in patients with ovarian cancer
- Affiliations
-
- 1Department of Obstetrics and Gynecology, Kaohsiung Veterans General Hospital, Kaohsiung, Taiwan, Republic of China.
- 2Department of Biological Sciences, National Sun Yat-sen University, Kaohsiung, Taiwan, Republic of China.
- 3Department of Pharmacy and Graduate Institute of Pharmaceutical Technology, Ta-Jen University, Pingtung, Taiwan, Republic of China.
- 4Multidisciplinary Science Research Center, Taiwan, Republic of China.
- 5Department of Applied Mathematics, National Sun Yat-sen University, Kaohsiung, Taiwan, Republic of China. cchang@math.nsysu.edu.tw
- KMID: 1811188
- DOI: http://doi.org/10.3802/jgo.2014.25.1.51
Abstract
OBJECTIVE
The objective of this study was to explore the association of longitudinal CA-125 measurements with overall survival (OS) time by developing a flexible model for patient-specific CA-125 profiles, and to provide a simple and reliable prediction of OS.
METHODS
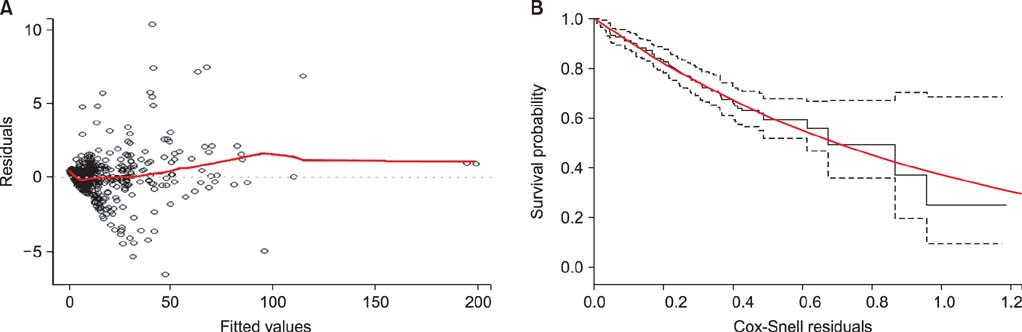
A retrospective study was performed on 275 patients with ovarian cancer who underwent at least one cycle of primary chemotherapy in our institute. Serial measurements of patients' CA-125 levels were performed at different frequencies according to their clinical plans. A statistical model coupling the Cox proportional hazards and the mixed-effects models was applied to determine the association of OS with patient-specific longitudinal CA-125 values. Stage and residual tumor size were additional variables included in the analysis.
RESULTS
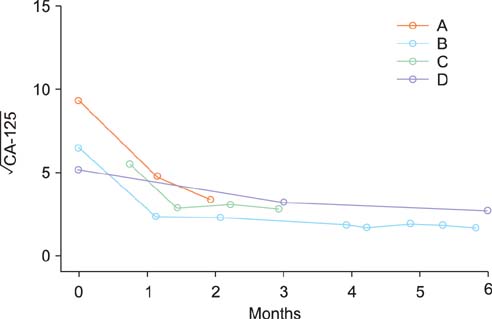
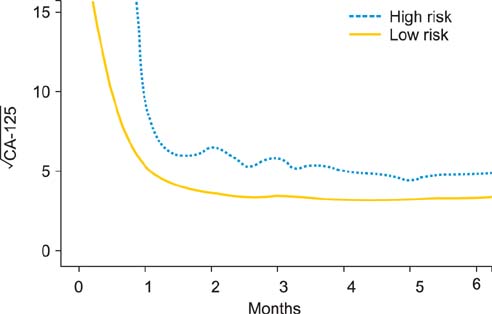
A total of 1,601 values of CA-125 were included. Longitudinal CA-125 levels, stage, and the residual tumor size were all significantly associated with OS. A patient-specific survival probability could be calculated. Validation showed that, in average, 85.4% patients were correctly predicted to have a high or low risk of death at a given time point. Comparison with a traditional model using CA-125 half-life and time to reach CA-125 nadir showed that the longitudinal CA-125 model had an improved predicative value.
CONCLUSION
Longitudinal CA-125 values, measured from the diagnosis of ovarian cancer to the completion of primary chemotherapy, could be used to reliably predict OS after adjusting for the stage and residual tumor disease. This model could be potentially useful in clinical counseling of patients with ovarian cancer.
MeSH Terms
Figure
Reference
-
1. Sankaranarayanan R, Ferlay J. Worldwide burden of gynaecological cancer: the size of the problem. Best Pract Res Clin Obstet Gynaecol. 2006; 20:207–225.2. Su Z, Graybill WS, Zhu Y. Detection and monitoring of ovarian cancer. Clin Chim Acta. 2013; 415:341–345.3. Markowska J, Manys G, Kubaszewska M. Value of CA 125 as a marker of ovarian cancer. Eur J Gynaecol Oncol. 1992; 13:360–365.4. Gupta D, Lis CG. Role of CA125 in predicting ovarian cancer survival: a review of the epidemiological literature. J Ovarian Res. 2009; 2:13.5. Boere IA, van der Burg ME. Review of dose-intense platinum and/or paclitaxel containing chemotherapy in advanced and recurrent epithelial ovarian cancer. Curr Pharm Des. 2012; 18:3741–3753.6. Rizopoulos D. Dynamic predictions and prospective accuracy in joint models for longitudinal and time-to-event data. Biometrics. 2011; 67:819–829.7. Polterauer S, Vergote I, Concin N, Braicu I, Chekerov R, Mahner S, et al. Prognostic value of residual tumor size in patients with epithelial ovarian cancer FIGO stages IIA-IV: analysis of the OVCAD data. Int J Gynecol Cancer. 2012; 22:380–385.8. R Development Core Team. R: a language and environment of statistical computing. Vienna, Austria: R Foundation for Statistical Computing;2010.9. Rizopoulos D, Verbeke G, Molenberghs G. Multiple-imputation-based residuals and diagnostic plots for joint models of longitudinal and survival outcomes. Biometrics. 2010; 66:20–29.10. Cox DR. Regression models and life-tables. J R Stat Soc Series B Stat Methodol. 1972; 34:187–220.11. Ying LS, Xu SH, Su D, Mou HZ, Gu LH, Zhu CH, et al. Study of the metastasis-associated genes and its copy numbers variation in highly metastatic epithelial ovarian cancer. Zhonghua Fu Chan Ke Za Zhi. 2009; 44:126–130.12. Cleveland WS. Robust locally weighted regression and smoothing scatterplots. J Am Stat Assoc. 1979; 74:829–836.13. Gadducci A, Cosio S, Fanucchi A, Negri S, Cristofani R, Genazzani AR. The predictive and prognostic value of serum CA 125 half-life during paclitaxel/platinum-based chemotherapy in patients with advanced ovarian carcinoma. Gynecol Oncol. 2004; 93:131–136.14. Riedinger JM, Wafflart J, Ricolleau G, Eche N, Larbre H, Basuyau JP, et al. CA 125 half-life and CA 125 nadir during induction chemotherapy are independent predictors of epithelial ovarian cancer outcome: results of a French multicentric study. Ann Oncol. 2006; 17:1234–1238.15. Nolen BM, Lokshin AE. Protein biomarkers of ovarian cancer: the forest and the trees. Future Oncol. 2012; 8:55–71.16. Díaz-Padilla I, Razak AR, Minig L, Bernardini MQ, Maria Del Campo J. Prognostic and predictive value of CA-125 in the primary treatment of epithelial ovarian cancer: potentials and pitfalls. Clin Transl Oncol. 2012; 14:15–20.17. Kim HS, Park NH, Chung HH, Kim JW, Song YS, Kang SB. Serum CA-125 level after 6 cycles of primary adjuvant chemotherapy is a useful prognostic factor for complete responders' survival in patients with advanced epithelial ovarian cancer. Onkologie. 2008; 31:315–320.18. Makar AP, Kristensen GB, Kaern J, Børmer OP, Abeler VM, Trope CG. Prognostic value of pre- and postoperative serum CA 125 levels in ovarian cancer: new aspects and multivariate analysis. Obstet Gynecol. 1992; 79:1002–1010.19. van Dalen A, Favier J, Burges A, Hasholzner U, de Bruijn HW, Dobler-Girdziunaite D, et al. Prognostic significance of CA 125 and TPS levels after 3 chemotherapy courses in ovarian cancer patients. Gynecol Oncol. 2000; 79:444–450.20. Ron IG, Inbar M, Gelernter I, Lewysohn O, Ayalon D, Dale J, et al. Use of CA-125 response to predict survival parameters of patients with advanced ovarian carcinoma. Acta Obstet Gynecol Scand. 1994; 73:658–662.21. Markman M, Federico M, Liu PY, Hannigan E, Alberts D. Significance of early changes in the serum CA-125 antigen level on overall survival in advanced ovarian cancer. Gynecol Oncol. 2006; 103:195–198.22. Osman N, O'Leary N, Mulcahy E, Barrett N, Wallis F, Hickey K, et al. Correlation of serum CA125 with stage, grade and survival of patients with epithelial ovarian cancer at a single centre. Ir Med J. 2008; 101:245–247.23. Bidart JM, Thuillier F, Augereau C, Chalas J, Daver A, Jacob N, et al. Kinetics of serum tumor marker concentrations and usefulness in clinical monitoring. Clin Chem. 1999; 45:1695–1707.24. Mano A, Falcao A, Godinho I, Santos J, Leitao F, de Oliveira C, et al. CA-125 AUC as a predictor for epithelial ovarian cancer relapse. Cancer Biomark. 2008; 4:73–81.25. Gupta D, Lammersfeld CA, Vashi PG, Braun DP. Longitudinal monitoring of CA125 levels provides additional information about survival in ovarian cancer. J Ovarian Res. 2010; 3:22.26. Riedinger JM, Bonnetain F, Basuyau JP, Eche N, Larbre H, Dalifard I, et al. Change in CA 125 levels after the first cycle of induction chemotherapy is an independent predictor of epithelial ovarian tumour outcome. Ann Oncol. 2007; 18:881–885.27. Goonetilleke KS, Siriwardena AK. Systematic review of carbohydrate antigen (CA 19-9) as a biochemical marker in the diagnosis of pancreatic cancer. Eur J Surg Oncol. 2007; 33:266–270.28. Shering SG, Sherry F, McDermott EW, O'Higgins NJ, Duffy MJ. Preoperative CA 15-3 concentrations predict outcome of patients with breast carcinoma. Cancer. 1998; 83:2521–2527.29. Høgdall EV, Christensen L, Kjaer SK, Blaakaer J, Kjaerbye-Thygesen A, Gayther S, et al. CA125 expression pattern, prognosis and correlation with serum CA125 in ovarian tumor patients. From The Danish "MALOVA" Ovarian Cancer Study. Gynecol Oncol. 2007; 104:508–515.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Use of CA 125 Antigen to Predict Suvival of Patients with Epithelial Ovarian Cancer
- A clinical evaluation of CA 125 antigen values in patients of ovarian cancer
- Serum CA 125 Levels in Patients with Impaired Renal Function
- Prognostic Value of Serum CA 125 Measurment during Chemotherapy for the Patients with Epithelial Ovarian Cancer
- Clinical Relevance of the CA 125 Assay in Monitoring of Epithelial Ovarian Cancer Patients