Diabetes Metab J.
2015 Apr;39(2):87-94. 10.4093/dmj.2015.39.2.87.
New Drugs for Treating Dyslipidemia: Beyond Statins
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea. drshchoi@snu.ac.kr
- KMID: 1805891
- DOI: http://doi.org/10.4093/dmj.2015.39.2.87
Abstract
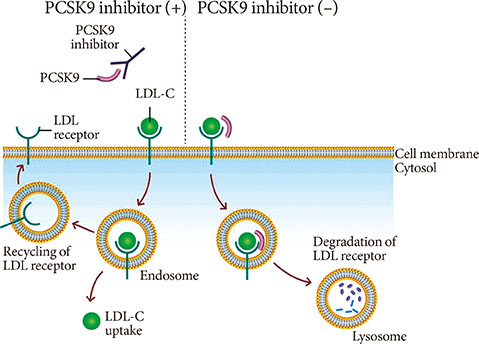
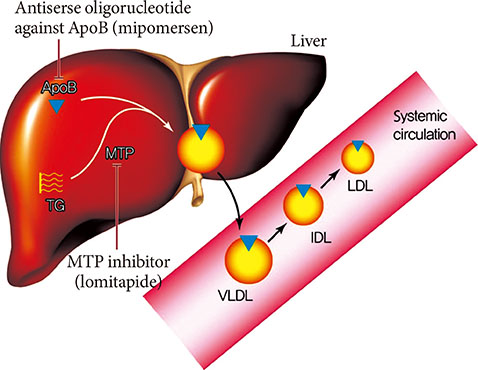
- Statins have been shown to be very effective and safe in numerous randomized clinical trials, and became the implacable first-line treatment against atherogenic dyslipidemia. However, even with optimal statin treatment, 60% to 80% of residual cardiovascular risk still exists. The patients with familial hypercholesterolemia which results in extremely high level of low density lipoprotein cholesterol (LDL-C) level and the patients who are intolerant or unresponsive to statins are the other hurdles of statin treatment. Recently, new classes of lipid-lowering drugs have been developed and some of them are available for the clinical practice. The pro-protein convertase subtilisin/kexintype 9 (PCSK9) inhibitor increases the expression of low density lipoprotein (LDL) receptor in hepatocytes by enhancing LDL receptor recycling. The microsomal triglyceride transport protein (MTP) inhibitor and antisense oligonucleotide against apolipoprotein B (ApoB) reduce the ApoB containing lipoprotein by blocking the hepatic very low density lipoprotein synthesis pathway. The apolipoprotein A1 (ApoA1) mimetics pursuing the beneficial effect of high density lipoprotein cholesterol and can reverse the course of atherosclerosis. ApoA1 mimetics had many controversial clinical data and need more validation in humans. The PCSK9 inhibitor recently showed promising results of significant LDL-C lowering in familial hypercholesterolemia (FH) patients from the long-term phase III trials. The MTP inhibitor and antisesnse oligonucleotide against ApoB were approved for the treatment of homozygous FH but still needs more consolidated evidences about hepatic safety such as hepatosteatosis. We would discuss the benefits and concerns of these new lipid-lowering drugs anticipating additional benefits beyond statin treatment.
Keyword
MeSH Terms
-
Apolipoprotein A-I
Apolipoproteins
Apolipoproteins B
Atherosclerosis
Cholesterol, HDL
Cholesterol, LDL
Dyslipidemias*
Hepatocytes
Humans
Hydroxymethylglutaryl-CoA Reductase Inhibitors*
Hyperlipoproteinemia Type II
Lipoproteins
Receptors, LDL
Recycling
Triglycerides
Apolipoprotein A-I
Apolipoproteins
Apolipoproteins B
Cholesterol, HDL
Cholesterol, LDL
Lipoproteins
Receptors, LDL
Figure
Cited by 2 articles
-
Pharmacological Strategies beyond Statins: Ezetimibe and PCSK9 Inhibitors
Jah Yeon Choi, Jin Oh Na
J Lipid Atheroscler. 2019;8(2):183-191. doi: 10.12997/jla.2019.8.2.183.New, Novel Lipid-Lowering Agents for Reducing Cardiovascular Risk: Beyond Statins
Kyuho Kim, Henry N. Ginsberg, Sung Hee Choi
Diabetes Metab J. 2022;46(4):517-532. doi: 10.4093/dmj.2022.0198.
Reference
-
1. Roh E, Ko SH, Kwon HS, Kim NH, Kim JH, Kim CS, Song KH, Won JC, Kim DJ, Choi SH, Lim S, Cha BY. Prevalence and Management of Dyslipidemia in Korea: Korea National Health and Nutrition Examination Survey during 1998 to 2010. Diabetes Metab J. 2013; 37:433–449.2. Koo BK. Statin for the primary prevention of cardiovascular disease in patients with diabetes mellitus. Diabetes Metab J. 2014; 38:32–34.3. Lim S, Park YM, Sakuma I, Koh KK. How to control residual cardiovascular risk despite statin treatment: focusing on HDL-cholesterol. Int J Cardiol. 2013; 166:8–14.4. Sampson UK, Fazio S, Linton MF. Residual cardiovascular risk despite optimal LDL cholesterol reduction with statins: the evidence, etiology, and therapeutic challenges. Curr Atheroscler Rep. 2012; 14:1–10.5. Hovingh GK, Davidson MH, Kastelein JJ, O'Connor AM. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J. 2013; 34:962–971.6. Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014; 384:607–617.7. DiNicolantonio JJ, Chatterjee S, Lavie CJ, Bangalore S, O'Keefe JH. Ezetimibe plus moderate dose simvastatin after acute coronary syndrome: what are we IMPROVEing on? Am J Med. Epub 2015 Feb 27. DOI: http://dx.doi.org/10.1016/j.amjmed.2015.01.034.8. Abifadel M, Varret M, Rabes JP, Allard D, Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich D, Derre A, Villeger L, Farnier M, Beucler I, Bruckert E, Chambaz J, Chanu B, Lecerf JM, Luc G, Moulin P, Weissenbach J, Prat A, Krempf M, Junien C, Seidah NG, Boileau C. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003; 34:154–156.9. Horton JD, Cohen JC, Hobbs HH. Molecular biology of PCSK9: its role in LDL metabolism. Trends Biochem Sci. 2007; 32:71–77.10. Do RQ, Vogel RA, Schwartz GG. PCSK9 Inhibitors: potential in cardiovascular therapeutics. Curr Cardiol Rep. 2013; 15:345.11. Noto D, Cefalu AB, Averna MR. Beyond statins: new lipid lowering strategies to reduce cardiovascular risk. Curr Atheroscler Rep. 2014; 16:414.12. Denis M, Marcinkiewicz J, Zaid A, Gauthier D, Poirier S, Lazure C, Seidah NG, Prat A. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012; 125:894–901.13. Al-Mashhadi RH, Sorensen CB, Kragh PM, Christoffersen C, Mortensen MB, Tolbod LP, Thim T, Du Y, Li J, Liu Y, Moldt B, Schmidt M, Vajta G, Larsen T, Purup S, Bolund L, Nielsen LB, Callesen H, Falk E, Mikkelsen JG, Bentzon JF. Familial hypercholesterolemia and atherosclerosis in cloned minipigs created by DNA transposition of a human PCSK9 gain-of-function mutant. Sci Transl Med. 2013; 5:166ra1.14. Stein EA, Gipe D, Bergeron J, Gaudet D, Weiss R, Dufour R, Wu R, Pordy R. Effect of a monoclonal antibody to PCSK9, REGN727/SAR236553, to reduce low-density lipoprotein cholesterol in patients with heterozygous familial hypercholesterolaemia on stable statin dose with or without ezetimibe therapy: a phase 2 randomised controlled trial. Lancet. 2012; 380:29–36.15. Sullivan D, Olsson AG, Scott R, Kim JB, Xue A, Gebski V, Wasserman SM, Stein EA. Effect of a monoclonal antibody to PCSK9 on low-density lipoprotein cholesterol levels in statin-intolerant patients: the GAUSS randomized trial. JAMA. 2012; 308:2497–2506.16. Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, Shahawy ME, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ. Efficacy and Safety of Alirocumab in Reducing Lipids and Cardiovascular Events. N Engl J Med. 2015.17. Sabatine MS, Giugliano RP, Wiviott SD, Raal FJ, Blom DJ, Robinson J, Ballantyne CM, Somaratne R, Legg J, Wasserman SM, Scott R, Koren MJ, Stein EA. Efficacy and Safety of Evolocumab in Reducing Lipids and Cardiovascular Events. N Engl J Med. 2015.18. Choi SH, Ginsberg HN. Increased very low density lipoprotein (VLDL) secretion, hepatic steatosis, and insulin resistance. Trends Endocrinol Metab. 2011; 22:353–363.19. Wetterau JR, Aggerbeck LP, Bouma ME, Eisenberg C, Munck A, Hermier M, Schmitz J, Gay G, Rader DJ, Gregg RE. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science. 1992; 258:999–1001.20. Cuchel M, Bloedon LT, Szapary PO, Kolansky DM, Wolfe ML, Sarkis A, Millar JS, Ikewaki K, Siegelman ES, Gregg RE, Rader DJ. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N Engl J Med. 2007; 356:148–156.21. Rader DJ, Kastelein JJ. Lomitapide and mipomersen: two first-in-class drugs for reducing low-density lipoprotein cholesterol in patients with homozygous familial hypercholesterolemia. Circulation. 2014; 129:1022–1032.22. Wetterau JR, Gregg RE, Harrity TW, Arbeeny C, Cap M, Connolly F, Chu CH, George RJ, Gordon DA, Jamil H, Jolibois KG, Kunselman LK, Lan SJ, Maccagnan TJ, Ricci B, Yan M, Young D, Chen Y, Fryszman OM, Logan JV, Musial CL, Poss MA, Robl JA, Simpkins LM, Slusarchyk WA, Sulsky R, Taunk P, Magnin DR, Tino JA, Lawrence RM, Dickson JK Jr, Biller SA. An MTP inhibitor that normalizes atherogenic lipoprotein levels in WHHL rabbits. Science. 1998; 282:751–754.23. Dhote V, Joharapurkar A, Kshirsagar S, Dhanesha N, Patel V, Patel A, Raval S, Jain M. Inhibition of microsomal triglyceride transfer protein improves insulin sensitivity and reduces atherogenic risk in Zucker fatty rats. Clin Exp Pharmacol Physiol. 2011; 38:338–344.24. Cuchel M, Meagher EA, du Toit, Blom DJ, Marais AD, Hegele RA, Averna MR, Sirtori CR, Shah PK, Gaudet D, Stefanutti C, Vigna GB, Du Plessis AM, Propert KJ, Sasiela WJ, Bloedon LT, Rader DJ. Phase 3 HoFH Lomitapide Study investigators. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolaemia: a single-arm, open-label, phase 3 study. Lancet. 2013; 381:40–46.25. Sahebkar A, Watts GF. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: what can the clinician expect? Cardiovasc Drugs Ther. 2013; 27:559–567.26. Kastelein JJ, Wedel MK, Baker BF, Su J, Bradley JD, Yu RZ, Chuang E, Graham MJ, Crooke RM. Potent reduction of apolipoprotein B and low-density lipoprotein cholesterol by short-term administration of an antisense inhibitor of apolipoprotein B. Circulation. 2006; 114:1729–1735.27. Akdim F, Visser ME, Tribble DL, Baker BF, Stroes ES, Yu R, Flaim JD, Su J, Stein EA, Kastelein JJ. Effect of mipomersen, an apolipoprotein B synthesis inhibitor, on low-density lipoprotein cholesterol in patients with familial hypercholesterolemia. Am J Cardiol. 2010; 105:1413–1419.28. Akdim F, Tribble DL, Flaim JD, Yu R, Su J, Geary RS, Baker BF, Fuhr R, Wedel MK, Kastelein JJ. Efficacy of apolipoprotein B synthesis inhibition in subjects with mild-to-moderate hyperlipidaemia. Eur Heart J. 2011; 32:2650–2659.29. Gordon T, Castelli WP, Hjortland MC, Kannel WB, Dawber TR. High density lipoprotein as a protective factor against coronary heart disease. The Framingham Study. Am J Med. 1977; 62:707–714.30. Sherman CB, Peterson SJ, Frishman WH. Apolipoprotein A-I mimetic peptides: a potential new therapy for the prevention of atherosclerosis. Cardiol Rev. 2010; 18:141–147.31. Smith JD. Apolipoprotein A-I and its mimetics for the treatment of atherosclerosis. Curr Opin Investig Drugs. 2010; 11:989–996.32. Franceschini G, Sirtori CR, Capurso A 2nd, Weisgraber KH, Mahley RW. A-IMilano apoprotein. Decreased high density lipoprotein cholesterol levels with significant lipoprotein modifications and without clinical atherosclerosis in an Italian family. J Clin Invest. 1980; 66:892–900.33. Gualandri V, Franceschini G, Sirtori CR, Gianfranceschi G, Orsini GB, Cerrone A, Menotti A. AIMilano apoprotein identification of the complete kindred and evidence of a dominant genetic transmission. Am J Hum Genet. 1985; 37:1083–1097.34. Parolini C, Marchesi M, Lorenzon P, Castano M, Balconi E, Miragoli L, Chaabane L, Morisetti A, Lorusso V, Martin BJ, Bisgaier CL, Krause B, Newton RS, Sirtori CR, Chiesa G. Dose-related effects of repeated ETC-216 (recombinant apolipoprotein A-I Milano/1-palmitoyl-2-oleoyl phosphatidylcholine complexes) administrations on rabbit lipid-rich soft plaques: in vivo assessment by intravascular ultrasound and magnetic resonance imaging. J Am Coll Cardiol. 2008; 51:1098–1103.35. Navab M, Anantharamaiah GM, Hama S, Garber DW, Chaddha M, Hough G, Lallone R, Fogelman AM. Oral administration of an Apo A-I mimetic peptide synthesized from D-amino acids dramatically reduces atherosclerosis in mice independent of plasma cholesterol. Circulation. 2002; 105:290–292.36. Li X, Chyu KY, Faria Neto JR, Yano J, Nathwani N, Ferreira C, Dimayuga PC, Cercek B, Kaul S, Shah PK. Differential effects of apolipoprotein A-I-mimetic peptide on evolving and established atherosclerosis in apolipoprotein E-null mice. Circulation. 2004; 110:1701–1705.37. Morgantini C, Imaizumi S, Grijalva V, Navab M, Fogelman AM, Reddy ST. Apolipoprotein A-I mimetic peptides prevent atherosclerosis development and reduce plaque inflammation in a murine model of diabetes. Diabetes. 2010; 59:3223–3228.38. Xie Q, Zhao SP, Li F. D-4F, an apolipoprotein A-I mimetic peptide, promotes cholesterol efflux from macrophages via ATP-binding cassette transporter A1. Tohoku J Exp Med. 2010; 220:223–228.39. Nissen SE, Tsunoda T, Tuzcu EM, Schoenhagen P, Cooper CJ, Yasin M, Eaton GM, Lauer MA, Sheldon WS, Grines CL, Halpern S, Crowe T, Blankenship JC, Kerensky R. Effect of recombinant ApoA-I Milano on coronary atherosclerosis in patients with acute coronary syndromes: a randomized controlled trial. JAMA. 2003; 290:2292–2300.40. Tardif JC, Gregoire J, L’Allier PL, Ibrahim R, Lesperance J, Heinonen TM, Kouz S, Berry C, Basser R, Lavoie MA, Guertin MC, Rodes-Cabau J. Effect of rHDL on Atherosclerosis-Safety and Efficacy (ERASE) Investigators. Effects of reconstituted high-density lipoprotein infusions on coronary atherosclerosis: a randomized controlled trial. JAMA. 2007; 297:1675–1682.41. Bloedon LT, Dunbar R, Duffy D, Pinell-Salles P, Norris R, DeGroot BJ, Movva R, Navab M, Fogelman AM, Rader DJ. Safety, pharmacokinetics, and pharmacodynamics of oral apoA-I mimetic peptide D-4F in high-risk cardiovascular patients. J Lipid Res. 2008; 49:1344–1352.42. Watson CE, Weissbach N, Kjems L, Ayalasomayajula S, Zhang Y, Chang I, Navab M, Hama S, Hough G, Reddy ST, Soffer D, Rader DJ, Fogelman AM, Schecter A. Treatment of patients with cardiovascular disease with L-4F, an apo-A1 mimetic, did not improve select biomarkers of HDL function. J Lipid Res. 2011; 52:361–373.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Management of Dyslipidemia after Stroke
- Practical Approaches to Managing Dyslipidemia in Patients With Metabolic Dysfunction-Associated Steatotic Liver Disease
- The Association between Medication Use for Dyslipidemia and Osteoporosis
- Update on the Pharmacologic Agents for Dyslipidemia
- Adverse effects of statin therapy and their treatment