J Korean Bone Joint Tumor Soc.
2014 Dec;20(2):66-73. 10.5292/jkbjts.2014.20.2.66.
Evaluation of Neoadjuvant Chemotherapy Effect in Osteosarcoma
- Affiliations
-
- 1Department of Orthopaedic Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea. ygchung@catholic.ac.kr
- 2Department of Radiology, College of Medicine, The Catholic University of Korea, Seoul, Korea.
- KMID: 1799895
- DOI: http://doi.org/10.5292/jkbjts.2014.20.2.66
Abstract
- PURPOSE
Various diagnostic imaging modalities have been used to evaluate the effect of neoadjuvant chemotherapy for osteosarcoma early and noninvasively. We evaluated the effectiveness of imaging studies of plain radiographs and positron-emission tomography/computed tomography (PET/CT) in predicting neoadjuvant chemotherapy effect for osteosarcoma and tried to establish a general principle in interpretation of PET/CT parameters.
MATERIALS AND METHODS
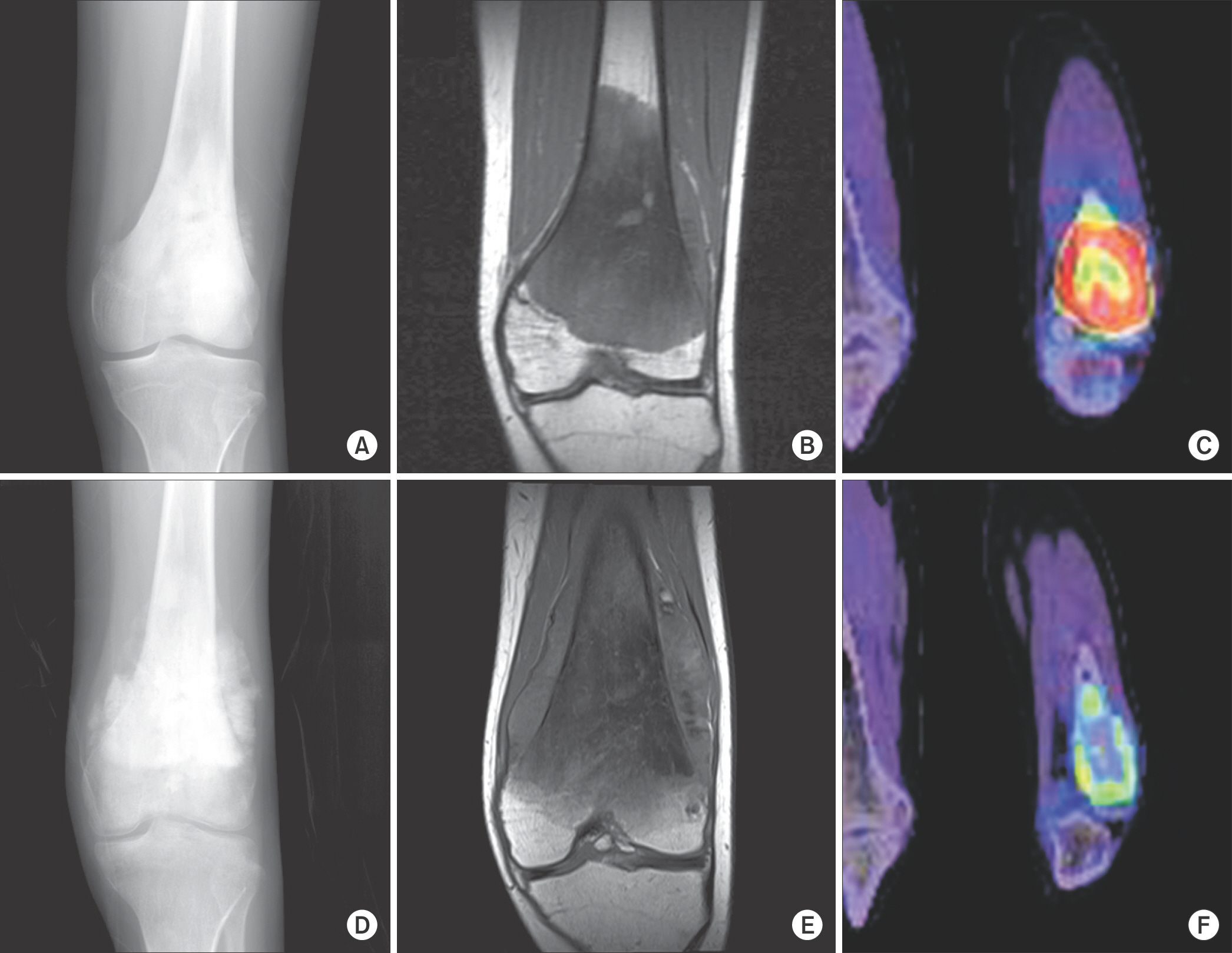
Eighteen patients who underwent two cycles of neoadjuvant chemotherapy and surgical excision for osteosarcoma were enrolled. There were 13 males and 5 females, with a median age of 19 (11-63) years. Fifteen patients of 18 had the American Joint Committe on Cancer (AJCC) stage IIB. They had plain radiographs and PET/CT before and after neoadjuvant chemotherapy. The resected tumor specimens were pathologically examined to determine histological response grade using a conventional mapping method. Statistical analysis was performed to evaluate the correlation between histopathological necrosis rate, and radiographic finding category, post-chemotherapy maximum standardized uptake value (SUVmax), average standardized uptake value and metabolic tumor volume (MTV) as well as reduction rates of them.
RESULTS
Eight patients were good responders to neoadjuvant chemotherapy based on histological evaluation. Median SUVmax reduction rate was 73 (23-77) % in good responders and 42 (-32-76) % in poor responders. Median MTV reduction rate was 93.5 (62-99) % in good responders and 46 (-81-100) % in poor responders. While radiographic finding category was not different according to histological response (p=1.0), SUVmax reduction rate was significantly different (p=0.041). Difference in MTV reduction rates approached statistical significance as well (p=0.071).
CONCLUSION
While radiographic finding category was not reliable to assess neoadjuvant chemotherapy effect for osteosarcoma, reduction rate of SUVmax was a useful indicator in this study. As parameters of PET/CT can be influenced by various factors of settings, different centers have to make an effort to establish their own standard of judgement with reference of previous studies.
MeSH Terms
Figure
Reference
-
References
1. Mirabello L, Troisi RJ, Savage SA. Osteosarcoma incidence and survival rates from 1973 to 2004: data from the Surveillance, Epidemiology, and End Results Program. Cancer. 2009; 115:1531–43.2. Bacci G, Longhi A, Fagioli F, Briccoli A, Versari M, Picci P. Adjuvant and neoadjuvant chemotherapy for osteosarcoma of the extremities: 27 year experience at Rizzoli Institute, Italy. Eur J Cancer. 2005; 41:2836–45.
Article3. Bacci G, Ferrari S, Bertoni F, et al. Long-term outcome for patients with nonmetastatic osteosarcoma of the extremity treated at the istituto ortopedico rizzoli according to the istituto ortopedico rizzoli/osteosarcoma-2 protocol: an updated report. J Clin Oncol. 2000; 18:4016–27.
Article4. Hagleitner MM, de Bont ES, Te Loo DM. Survival trends and longterm toxicity in pediatric patients with osteosarcoma. Sarcoma. 2012; 2012:636405.
Article5. Kim MS, Lee SY, Lee TR, et al. Prognostic nomogram for predicting the 5-year probability of developing metastasis after neoadjuvant chemotherapy and definitive surgery for AJCC stage II extremity osteosarcoma. Ann Oncol. 2009; 20:955–60.
Article6. Bajpai J, Gamnagatti S, Kumar R, et al. Role of MRI in osteosarcoma for evaluation and prediction of chemotherapy response: correlation with histological necrosis. Pediatr Radiol. 2011; 41:441–50.
Article7. Jeon DG, Song WS. How can survival be improved in localized osteosarcoma? Expert Rev Anticancer Ther. 2010; 10:1313–25.
Article8. Benz MR, Czernin J, Tap WD, et al. FDG-PET/CT Imaging Predicts Histopathologic Treatment Responses after Neoadjuvant Therapy in Adult Primary Bone Sarcomas. Sarcoma. 2010; 2010:143540.
Article9. Denecke T, Hundsdörfer P, Misch D, et al. Assessment of histological response of paediatric bone sarcomas using FDG PET in comparison to morphological volume measurement and standardized MRI parameters. Eur J Nucl Med Mol Imaging. 2010; 37:1842–53.
Article10. Franzius C, Sciuk J, Brinkschmidt C, Jürgens H, Schober O. Evaluation of chemotherapy response in primary bone tumors with F-18 FDG positron emission tomography compared with histologically assessed tumor necrosis. Clin Nucl Med. 2000; 25:874–81.
Article11. Hamada K, Tomita Y, Inoue A, et al. Evaluation of chemotherapy response in osteosarcoma with FDG-PET. Ann Nucl Med. 2009; 23:89–95.
Article12. Jones DN, McCowage GB, Sostman HD, et al. Monitoring of neoadjuvant therapy response of soft-tissue and musculoskeletal sarcoma using fluorine-18-FDG PET. J Nucl Med. 1996; 37:1438–44.13. Tateishi U, Kawai A, Chuman H, et al. PET/CT allows stratification of responders to neoadjuvant chemotherapy for high-grade sarcoma: a prospective study. Clin Nucl Med. 2011; 36:526–32.14. Holscher HC, Hermans J, Nooy MA, Taminiau AH, Hogen-doorn PC, Bloem JL. Can conventional radiographs be used to monitor the effect of neoadjuvant chemotherapy in patients with osteogenic sarcoma? Skeletal Radiol. 1996; 25:19–24.
Article15. Cheon GJ, Kim MS, Lee JA, et al. Prediction model of chemotherapy response in osteosarcoma by 18F-FDG PET and MRI. J Nucl Med. 2009; 50:1435–40.
Article16. Caldarella C, Salsano M, Isgrò MA, Treglia G. The Role of Fluorine-18-fluorodeoxyglucose Positron Emission Tomography in Assessing the Response to Neoadjuvant Treatment in Patients with Osteosarcoma. Int J Mol Imaging. 2012; 2012:870301.
Article17. Hongtao L, Hui Z, Bingshun W, et al. 18F-FDG positron emission tomography for the assessment of histological response to neoadjuvant chemotherapy in osteosarcomas: a metaanalysis. Surg Oncol. 2012; 21:e165–70.
Article18. Picci P, Bacci G, Campanacci M, et al. Histologic evaluation of necrosis in osteosarcoma induced by chemotherapy. Regional mapping of viable and nonviable tumor. Cancer. 1985; 56:1515–21.
Article19. Picci P, Sangiorgi L, Rougraff BT, Neff JR, Casadei R, Campanacci M. Relationship of chemotherapy-induced necrosis and surgical margins to local recurrence in osteosarcoma. J Clin Oncol. 1994; 12:2699–705.
Article20. Greco C, Rosenzweig K, Cascini GL, Tamburrini O. Current status of PET/CT for tumour volume definition in radiotherapy treatment planning for nonsmall cell lung cancer (NSCLC). Lung Cancer. 2007; 57:125–34.
Article21. Konski A, Doss M, Milestone B, et al. The integration of 18-fluoro-deoxy-glucose positron emission tomography and endoscopic ultrasound in the treatment-planning process for esophageal carcinoma. Int J Radiat Oncol Biol Phys. 2005; 61:1123–8.
Article22. Nestle U, Kremp S, Schaefer-Schuler A, et al. Comparison of different methods for delineation of 18F-FDG PET-positive tissue for target volume definition in radiotherapy of patients with non-Small cell lung cancer. J Nucl Med. 2005; 46:1342–8.23. Smith J, Heelan RT, Huvos AG, et al. Radiographic changes in primary osteogenic sarcoma following intensive chemotherapy. Radiological-pathological correlation in 63 patients. Radiology. 1982; 143:355–60.
Article24. Vanderhoek M, Perlman SB, Jeraj R. Impact of the definition of peak standardized uptake value on quantification of treatment response. J Nucl Med. 2012; 53:4–11.
Article25. Velasquez LM, Boellaard R, Kollia G, et al. Repeatability of 18F-FDG PET in a multicenter phase I study of patients with advanced gastrointestinal malignancies. J Nucl Med. 2009; 50:1646–54.
Article26. Wahl RL, Jacene H, Kasamon Y, Lodge MA. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J Nucl Med. 2009; 50(Suppl 1):122S–50S.
Article27. Im HJ, Kim TS, Park SY, et al. Prediction of tumour necrosis fractions using metabolic and volumetric 18F-FDG PET/CT indices, after one course and at the completion of neoadjuvant chemotherapy, in children and young adults with osteosarcoma. Eur J Nucl Med Mol Imaging. 2012; 39:39–49.
Article28. Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002; 20:776–90.
Article