Yonsei Med J.
2013 Nov;54(6):1422-1429. 10.3349/ymj.2013.54.6.1422.
Decreased Expression of Surfactant Protein Genes Is Associated with an Increased Expression of Forkhead Box M1 Gene in the Fetal Lung Tissues of Premature Rabbits
- Affiliations
-
- 1Department of Pediatrics, School of Medicine, Soonchunhyang University, Cheonan, Korea.
- 2Department of Pediatrics, School of Medicine, Kyung Hee University, Seoul, Korea. baecw@khnmc.or.kr
- KMID: 1798139
- DOI: http://doi.org/10.3349/ymj.2013.54.6.1422
Abstract
- PURPOSE
Recently, Forkhead box M1 (FoxM1) was reported to be correlated with lung maturation and expression of surfactant proteins (SPs) in mice models. However, no study has been conducted in rabbit lungs despite their high homology with human lungs. Thus, we attempted to investigate serial changes in the expressions of FoxM1 and SP-A/B throughout lung maturation in rabbit fetuses.
MATERIALS AND METHODS
Pregnant New Zealand White rabbits were grouped according to gestational age from 5 days before to 2 days after the day of expected full term delivery (F5, F4, F3, F2, F1, F0, P1, and P2). A total of 64 fetuses were enrolled after Cesarean sections. The expressions of mRNA and proteins of FoxM1 and SP-A/B in fetal lung tissue were tested by quantitative reverse-transcriptase real-time PCR and Western blot. Furthermore, their correlations were analyzed.
RESULTS
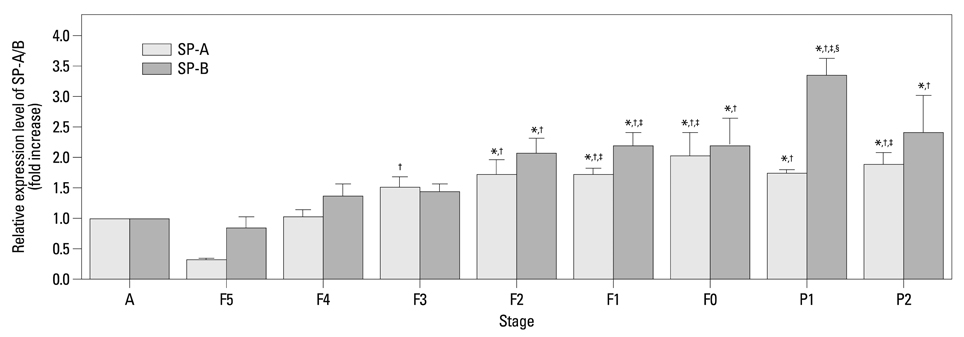
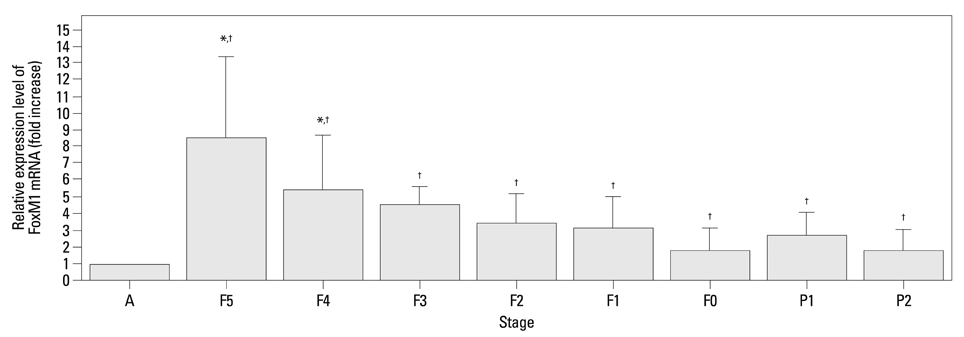
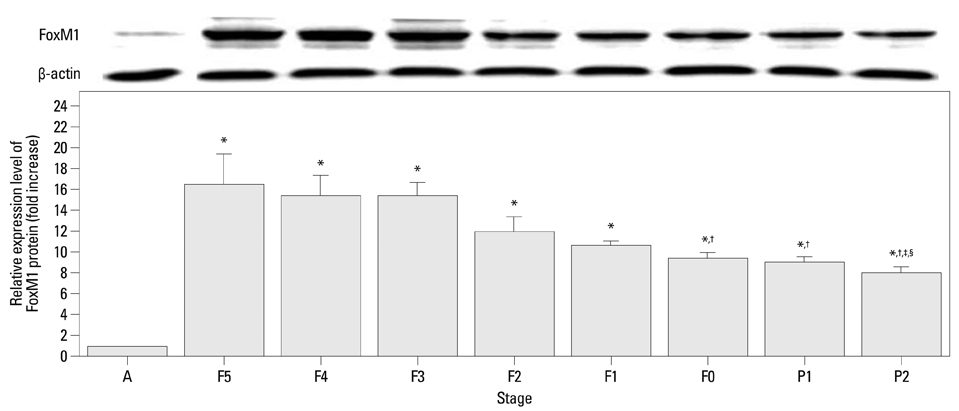
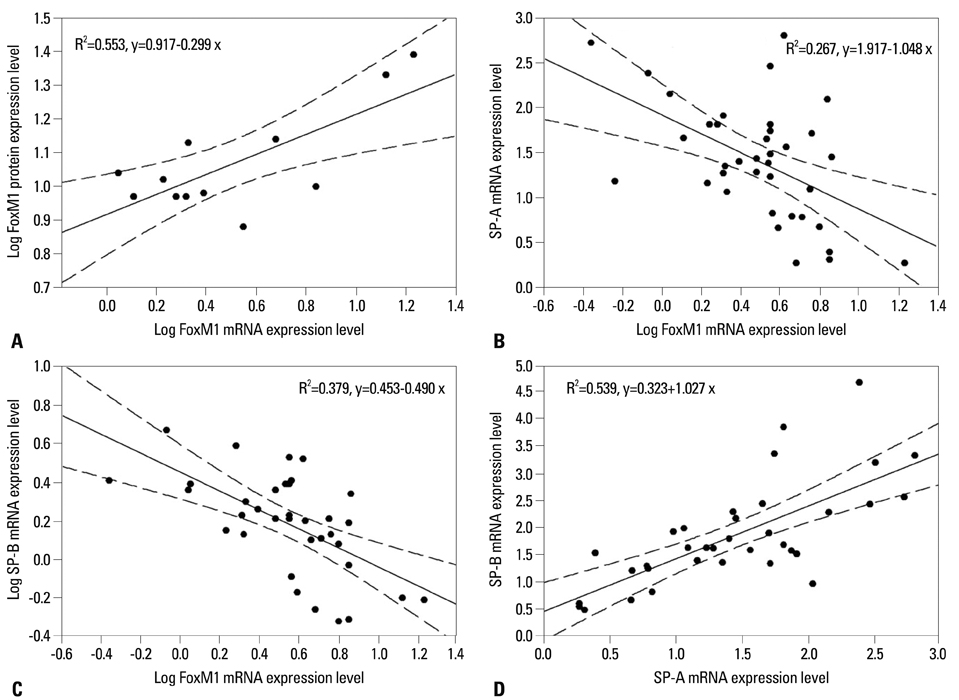
The mRNA expression of SP-A/B showed an increasing tendency positively correlated with gestational age, while the expression of FoxM1 mRNA and protein decreased from F5 to F0. A significant negative correlation was found between the expression levels of FoxM1 and SP-A/B (SP-A: R=-0.517, p=0.001; SP-B: R=-0.615, p<0.001).
CONCLUSION
Preterm rabbits demonstrated high expression of FoxM1 mRNA and protein in the lungs compared to full term rabbits. Also, the expression of SP-A/B was inversely related with serial changes in FoxM1 expression. This is the first report to suggest an association between FoxM1 and expression of SP-A/B and lung maturation in preterm rabbits.
Keyword
MeSH Terms
Figure
Reference
-
1. Costa RH, Kalinichenko VV, Lim L. Transcription factors in mouse lung development and function. Am J Physiol Lung Cell Mol Physiol. 2001; 280:L823–L838.
Article2. Wang X, Hung NJ, Costa RH. Earlier expression of the transcription factor HFH-11B diminishes induction of p21(CIP1/WAF1) levels and accelerates mouse hepatocyte entry into S-phase following carbon tetrachloride liver injury. Hepatology. 2001; 33:1404–1414.
Article3. Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A. 2002; 99:16881–16886.
Article4. Wang X, Quail E, Hung NJ, Tan Y, Ye H, Costa RH. Increased levels of forkhead box M1B transcription factor in transgenic mouse hepatocytes prevent age-related proliferation defects in regenerating liver. Proc Natl Acad Sci U S A. 2001; 98:11468–11473.
Article5. Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol. 1999; 19:8570–8580.
Article6. Ye H, Kelly TF, Samadani U, Lim L, Rubio S, Overdier DG, et al. Hepatocyte nuclear factor 3/fork head homolog 11 is expressed in proliferating epithelial and mesenchymal cells of embryonic and adult tissues. Mol Cell Biol. 1997; 17:1626–1641.
Article7. Carlsson P, Mahlapuu M. Forkhead transcription factors: key players in development and metabolism. Dev Biol. 2002; 250:1–23.
Article8. Zaret KS. Regulatory phases of early liver development: paradigms of organogenesis. Nat Rev Genet. 2002; 3:499–512.
Article9. Korver W, Roose J, Clevers H. The winged-helix transcription factor Trident is expressed in cycling cells. Nucleic Acids Res. 1997; 25:1715–1719.
Article10. Lüscher-Firzlaff JM, Westendorf JM, Zwicker J, Burkhardt H, Henriksson M, Müller R, et al. Interaction of the fork head domain transcription factor MPP2 with the human papilloma virus 16 E7 protein: enhancement of transformation and transactivation. Oncogene. 1999; 18:5620–5630.
Article11. Yao KM, Sha M, Lu Z, Wong GG. Molecular analysis of a novel winged helix protein, WIN. Expression pattern, DNA binding property, and alternative splicing within the DNA binding domain. J Biol Chem. 1997; 272:19827–19836.12. Wierstra I, Alves J. FOXM1, a typical proliferation-associated transcription factor. Biol Chem. 2007; 388:1257–1274.
Article13. Kim IM, Ramakrishna S, Gusarova GA, Yoder HM, Costa RH, Kalinichenko VV. The forkhead box m1 transcription factor is essential for embryonic development of pulmonary vasculature. J Biol Chem. 2005; 280:22278–22286.
Article14. Korver W, Schilham MW, Moerer P, van den Hoff MJ, Dam K, Lamers WH, et al. Uncoupling of S phase and mitosis in cardiomyocytes and hepatocytes lacking the winged-helix transcription factor Trident. Curr Biol. 1998; 8:1327–1330.
Article15. Krupczak-Hollis K, Wang X, Kalinichenko VV, Gusarova GA, Wang IC, Dennewitz MB, et al. The mouse Forkhead Box m1 transcription factor is essential for hepatoblast mitosis and development of intrahepatic bile ducts and vessels during liver morphogenesis. Dev Biol. 2004; 276:74–88.
Article16. Laoukili J, Kooistra MR, Brás A, Kauw J, Kerkhoven RM, Morrison A, et al. FoxM1 is required for execution of the mitotic programme and chromosome stability. Nat Cell Biol. 2005; 7:126–136.
Article17. Costa RH, Kalinichenko VV, Major ML, Raychaudhuri P. New and unexpected: forkhead meets ARF. Curr Opin Genet Dev. 2005; 15:42–48.
Article18. Kalin TV, Wang IC, Meliton L, Zhang Y, Wert SE, Ren X, et al. Forkhead Box m1 transcription factor is required for perinatal lung function. Proc Natl Acad Sci U S A. 2008; 105:19330–19335.
Article19. Chang JY. The Comparison of Forkhead Box M1 mRNA expression between preterm and term rabbit lung models (dissertation). Seoul: Hanyang Univ;2011.20. Durham PL, Nanthakumar EJ, Snyder JM. Developmental regulation of surfactant-associated proteins in rabbit fetal lung in vivo. Exp Lung Res. 1992; 18:775–793.
Article21. Kovar J, Sly PD, Willet KE. Postnatal alveolar development of the rabbit. J Appl Physiol. 2002; 93:629–635.
Article22. Starcher BC. Lung elastin and matrix. Chest. 2000; 117:5 Suppl 1. 229S–234S.
Article23. Korstanje R, O'Brien PC, Yang F, Rens W, Bosma AA, van Lith HA, et al. Complete homology maps of the rabbit (Oryctolagus cuniculus) and human by reciprocal chromosome painting. Cytogenet Cell Genet. 1999; 86:317–322.
Article24. Seppänen O, Glumoff V, Paananen R, Rounioja S, Hallman M. Transcription factors NF-kappaB and C/EBPdelta and IL-1-induced expression of surfactant protein A in lung explants during the perinatal period. Biol Neonate. 2005; 87:152–159.
Article25. Gras-Le Guen C, Denis C, Franco-Montoya ML, Jarry A, Delacourt C, Potel G, et al. Antenatal infection in the rabbit impairs post-natal growth and lung alveolarisation. Eur Respir J. 2008; 32:1520–1528.
Article26. Kaushal S, Ghosh S, Sharma N, Sanyal SN, Majumdar S. Role of phospholipid transfer protein in rabbit lung development. Cell Mol Life Sci. 2001; 58:2098–2107.
Article27. Roubliova XI, Van der Biest AM, Vaast P, Lu H, Jani JC, Lewi PJ, et al. Effect of maternal administration of betamethasone on peripheral arterial development in fetal rabbit lungs. Neonatology. 2008; 93:64–72.
Article28. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001; 25:402–408.
Article29. Kalinichenko VV, Gusarova GA, Tan Y, Wang IC, Major ML, Wang X, et al. Ubiquitous expression of the forkhead box M1B transgene accelerates proliferation of distinct pulmonary cell types following lung injury. J Biol Chem. 2003; 278:37888–37894.
Article30. Wang IC, Zhang Y, Snyder J, Sutherland MJ, Burhans MS, Shannon JM, et al. Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev Biol. 2010; 347:301–314.
Article31. Burri PH. The postnatal growth of the rat lung. 3. Morphology. Anat Rec. 1974; 180:77–98.32. Burri PH. Postnatal growth and maturation of the lung. Chest. 1975; 67:2 Suppl. 2S–3S.
Article33. Davies P, Reid L, Lister G, Pitt B. Postnatal growth of the sheep lung: a morphometric study. Anat Rec. 1988; 220:281–286.
Article34. Schittny JC, Burri PH. Development and growth of the lung. In : Fishman A, Elias J, Fishman J, Grippi M, Senior R, Pack A, editors. Fishman's pulmonary diseases and disorders. 4th ed. New-York: McGraw Hill Companies Inc;2008. p. 91–114.35. Mendelson CR. Role of transcription factors in fetal lung development and surfactant protein gene expression. Annu Rev Physiol. 2000; 62:875–915.
Article36. Duranthon V, Beaujean N, Brunner M, Odening KE, Santos AN, Kacskovics I, et al. On the emerging role of rabbit as human disease model and the instrumental role of novel transgenic tools. Transgenic Res. 2012; 21:699–713.
Article37. Fan J, Challah M, Watanabe T. Transgenic rabbit models for biomedical research: current status, basic methods and future perspectives. Pathol Int. 1999; 49:583–594.
Article38. Fan J, Watanabe T. Transgenic rabbits as therapeutic protein bioreactors and human disease models. Pharmacol Ther. 2003; 99:261–282.
Article39. Kikkawa Y, Motoyama EK, Gluck L. Study of the lungs of fetal and newborn rabbits. Morphologic, biochemical, and surface physical development. Am J Pathol. 1968; 52:177–210.40. Berg DK, Smith CS, Pearton DJ, Wells DN, Broadhurst R, Donnison M, et al. Trophectoderm lineage determination in cattle. Dev Cell. 2011; 20:244–255.
Article41. Zhao YY, Gao XP, Zhao YD, Mirza MK, Frey RS, Kalinichenko VV, et al. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006; 116:2333–2343.
Article42. Liu Y, Sadikot RT, Adami GR, Kalinichenko VV, Pendyala S, Natarajan V, et al. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med. 2011; 208:1473–1484.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Circadian Rhythm of Surfactant Protein A, B and C mRNA in Rats
- Circadian rhythm of surfactant protein A, B and C mRNA in rats
- Gene variant profiles and tumor metabolic activity as measured by FOXM1 expression and glucose uptake in lung adenocarcinoma
- The Effect of Endotoxin on Gene Expression and Total Amount of Surfactant Protein A
- Gene Expression of Surfactant Protein A mRNA of the Lung in Endotoxin and Thiourea Treated Rats