Yonsei Med J.
2013 Nov;54(6):1362-1369. 10.3349/ymj.2013.54.6.1362.
Dipeptidyl Peptidase 10, a Novel Prognostic Marker in Colorectal Cancer
- Affiliations
-
- 1Department of Pathology, Gangnam Severance Hospital, Yonsei University College of Medicine, Seoul, Korea.
- 2Colorectal Cancer Branch, Division of Translational and Clinical Research I, Research Institute and Hospital, National Cancer Center, Goyang, Korea. heejincmd@yahoo.com
- 3Center for Colorectal Cancer, Research Institute, National Cancer Center, Goyang, Korea.
- KMID: 1798131
- DOI: http://doi.org/10.3349/ymj.2013.54.6.1362
Abstract
- PURPOSE
The dipeptidyl peptidase IV (DPPIV) gene family exhibits multiple functions and is involved in the pathogenesis of various diseases. It has attracted pharmaceutical interest in the areas of metabolic disorders as well as cancer. However, clinicopathologic significance of DPPIV family in colorectal cancer is not fully understood.
MATERIALS AND METHODS
The clinical relevance of DPPIV and DPP10 expression was determined by immunohistochemical staining, and by assessing its clinicopathologic correlation in 383 colorectal cancer patients with known clinical outcomes.
RESULTS
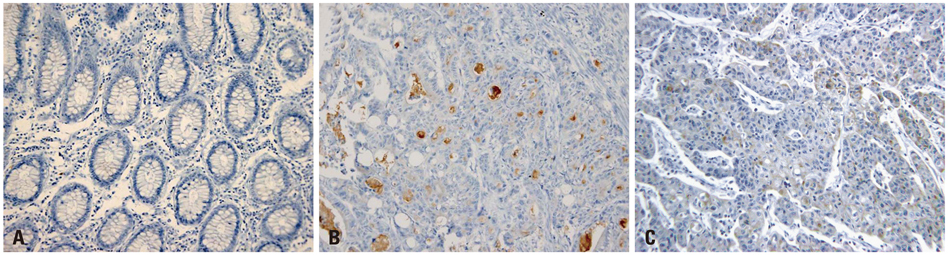
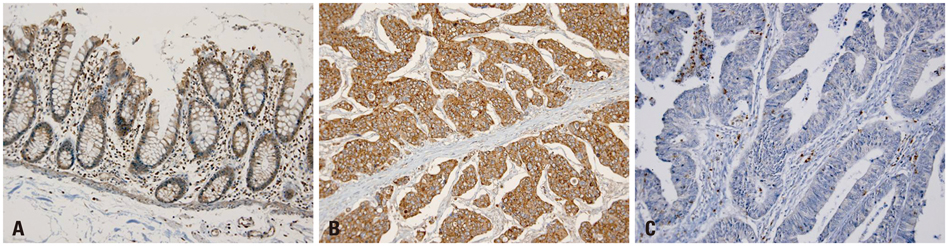
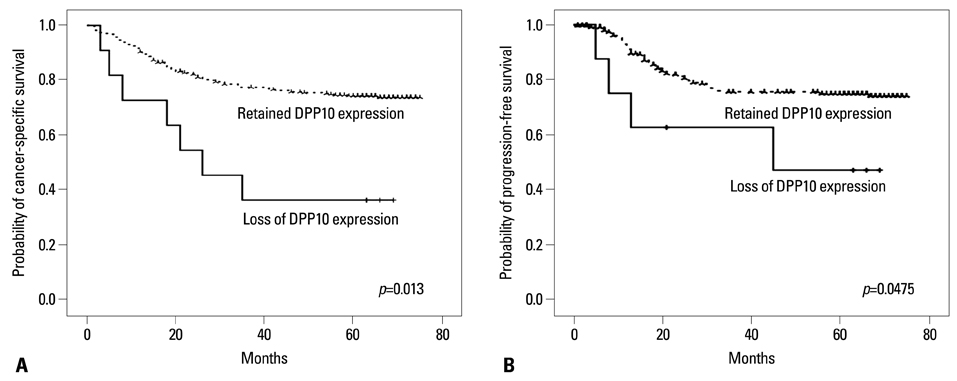
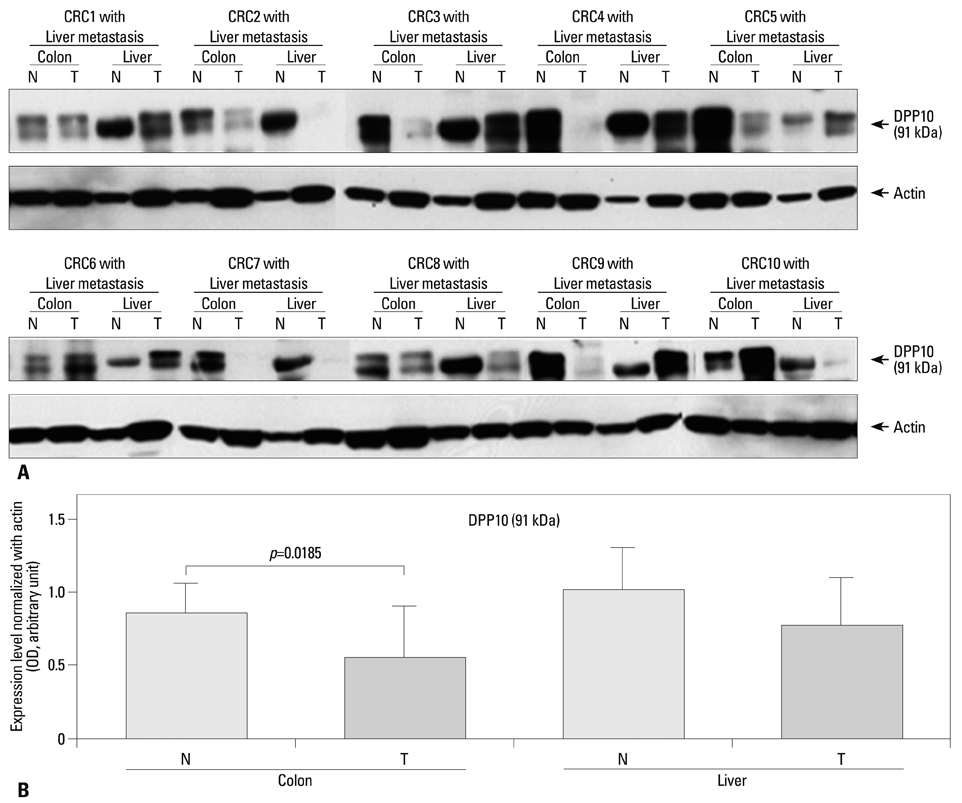
DPPIV was not expressed in normal colon mucosa, but it showed luminal expression in 52 of the 383 colorectal cancers (13.5%). DPPIV expression in tumors was associated with right-sided location of the colon (p=0.010) and more advanced tumor stage (p=0.045). DPP10 was expressed in normal colonic mucosa, but its expression varied in primary colorectal cancer tissues. Loss of DPP10 expression was found in 11 colorectal cancers (CRCs) (2.9%), and multivariate analysis showed that loss of DPP10 expression was an independent factor for poor patient prognosis (p=0.008).
CONCLUSION
DPP10 may play a role in disease progression of colorectal cancer and loss of DPP10 expression in primary CRC is significantly associated with poor survival outcomes.
MeSH Terms
Figure
Reference
-
1. Rastogi T, Hildesheim A, Sinha R. Opportunities for cancer epidemiology in developing countries. Nat Rev Cancer. 2004; 4:909–917.
Article2. Korea Central Cancer Resitry MoHaW, Republic of Korea. 2008 Annual Report of the Korea Central Cancer Registry. 2010.3. Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011; 61:212–236.4. Bacolod MD, Barany F. Molecular profiling of colon tumors: the search for clinically relevant biomarkers of progression, prognosis, therapeutics, and predisposition. Ann Surg Oncol. 2011; 18:3694–3700.
Article5. Yu DM, Yao TW, Chowdhury S, Nadvi NA, Osborne B, Church WB, et al. The dipeptidyl peptidase IV family in cancer and cell biology. FEBS J. 2010; 277:1126–1144.
Article6. Zagha E, Ozaita A, Chang SY, Nadal MS, Lin U, Saganich MJ, et al. DPP10 modulates Kv4-mediated A-type potassium channels. J Biol Chem. 2005; 280:18853–18861.
Article7. HUGO/GNC. Available at http://www.geneontology.org.8. Wesley UV, Albino AP, Tiwari S, Houghton AN. A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells. J Exp Med. 1999; 190:311–322.
Article9. Boonacker E, Van Noorden CJ. The multifunctional or moonlighting protein CD26/DPPIV. Eur J Cell Biol. 2003; 82:53–73.
Article10. Wesley UV, Tiwari S, Houghton AN. Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells. Int J Cancer. 2004; 109:855–866.
Article11. Sedo A, Krepela E, Kasafírek E, Kraml J, Kadlecová L. Dipeptidyl peptidase IV in the human lung and spinocellular lung cancer. Physiol Res. 1991; 40:359–362.12. Khin EE, Kikkawa F, Ino K, Kajiyama H, Suzuki T, Shibata K, et al. Dipeptidyl peptidase IV expression in endometrial endometrioid adenocarcinoma and its inverse correlation with tumor grade. Am J Obstet Gynecol. 2003; 188:670–676.
Article13. Tan EY, Mujoomdar M, Blay J. Adenosine down-regulates the surface expression of dipeptidyl peptidase IV on HT-29 human colorectal carcinoma cells: implications for cancer cell behavior. Am J Pathol. 2004; 165:319–330.
Article14. Qi SY, Riviere PJ, Trojnar J, Junien JL, Akinsanya KO. Cloning and characterization of dipeptidyl peptidase 10, a new member of an emerging subgroup of serine proteases. Biochem J. 2003; 373(Pt 1):179–189.
Article15. Li HL, Qu YJ, Lu YC, Bondarenko VE, Wang S, Skerrett IM, et al. DPP10 is an inactivation modulatory protein of Kv4.3 and Kv1.4. Am J Physiol Cell Physiol. 2006; 291:C966–C976.
Article16. Michel S, Liang L, Depner M, Klopp N, Ruether A, Kumar A, et al. Unifying candidate gene and GWAS Approaches in Asthma. PLoS One. 2010; 5:e13894.
Article17. Wu H, Romieu I, Shi M, Hancock DB, Li H, Sienra-Monge JJ, et al. Evaluation of candidate genes in a genome-wide association study of childhood asthma in Mexicans. J Allergy Clin Immunol. 2010; 125:321–327.
Article18. Bueno R, De Rienzo A, Dong L, Gordon GJ, Hercus CF, Richards WG, et al. Second generation sequencing of the mesothelioma tumor genome. PLoS One. 2010; 5:e10612.
Article19. Xiong S, Wang Q, Zheng L, Gao F, Li J. Identification of candidate molecular markers of nasopharyngeal carcinoma by tissue microarray and in situ hybridization. Med Oncol. 2011; 28:Suppl 1. S341–S348.
Article20. Dowsett M, Allred C, Knox J, Quinn E, Salter J, Wale C, et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol. 2008; 26:1059–1065.
Article21. Boonacker EP, Wierenga EA, Smits HH, Van Noorden CJ. CD26/DPPIV signal transduction function, but not proteolytic activity, is directly related to its expression level on human Th1 and Th2 cell lines as detected with living cell cytochemistry. J Histochem Cytochem. 2002; 50:1169–1177.
Article22. Allen M, Heinzmann A, Noguchi E, Abecasis G, Broxholme J, Ponting CP, et al. Positional cloning of a novel gene influencing asthma from chromosome 2q14. Nat Genet. 2003; 35:258–263.
Article23. McCaughan GW, Wickson JE, Creswick PF, Gorrell MD. Identification of the bile canalicular cell surface molecule GP110 as the ectopeptidase dipeptidyl peptidase IV: an analysis by tissue distribution, purification and N-terminal amino acid sequence. Hepatology. 1990; 11:534–544.
Article24. Boonacker E, Elferink S, Bardai A, Fleischer B, Van Noorden CJ. Fluorogenic substrate [Ala-Pro]2-cresyl violet but not Ala-Pro-rhodamine 110 is cleaved specifically by DPPIV activity: a study in living Jurkat cells and CD26/DPPIV-transfected Jurkat cells. J Histochem Cytochem. 2003; 51:959–968.
Article25. Asada Y, Aratake Y, Kotani T, Marutsuka K, Araki Y, Ohtaki S, et al. Expression of dipeptidyl aminopeptidase IV activity in human lung carcinoma. Histopathology. 1993; 23:265–270.
Article26. Wilson MJ, Ruhland AR, Quast BJ, Reddy PK, Ewing SL, Sinha AA. Dipeptidylpeptidase IV activities are elevated in prostate cancers and adjacent benign hyperplastic glands. J Androl. 2000; 21:220–226.27. Aratake Y, Kotani T, Tamura K, Araki Y, Kuribayashi T, Konoe K, et al. Dipeptidyl aminopeptidase IV staining of cytologic preparations to distinguish benign from malignant thyroid diseases. Am J Clin Pathol. 1991; 96:306–310.
Article28. ten Kate J, van den Ingh HF, Khan PM, Bosman FT. Adenosine deaminase complexing protein (ADCP) immunoreactivity in colorectal adenocarcinoma. Int J Cancer. 1986; 37:479–485.
Article29. Tao S, Haug U, Kuhn K, Brenner H. Comparison and combination of blood-based inflammatory markers with faecal occult blood tests for non-invasive colorectal cancer screening. Br J Cancer. 2012; 106:1424–1430.
Article30. Fric P, Sovová V, Sloncová E, Lojda Z, Jirásek A, Cermák J. Different expression of some molecular markers in sporadic cancer of the left and right colon. Eur J Cancer Prev. 2000; 9:265–268.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Letter: Association between Serum Dipeptidyl Peptidase-4 Concentration and Obesity-Related Factors in Health Screen Examinees (J Obes Metab Syndr 2017;26:188-96)
- Novel Therapies for Type 2 Diabetes Mellitus
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- Response: Association between Serum Dipeptidyl Peptidase-4 Concentration and Obesity-Related Factors in Health Screen Examinees (J Obes Metab Syndr 2017;26:188-96)