J Korean Med Sci.
2012 Mar;27(3):291-299. 10.3346/jkms.2012.27.3.291.
Increased Expression of EMMPRIN and VEGF in the Rat Brain after Gamma Irradiation
- Affiliations
-
- 1Department of Neurosurgery, the Second Hospital of Tianjin Medical University, Tianjin, China. neuroli@126.com
- 2Department of Neurosurgery, Tianjin Huanhu Hospital, and Tianjin Neurosurgery Institute, Tianjin, China.
- KMID: 1793001
- DOI: http://doi.org/10.3346/jkms.2012.27.3.291
Abstract
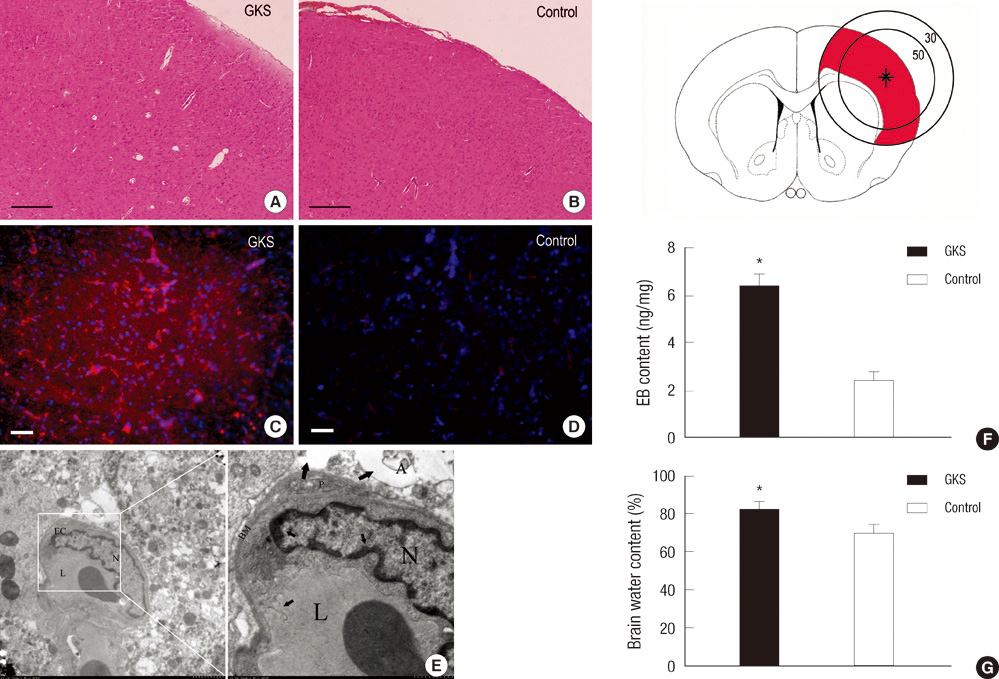
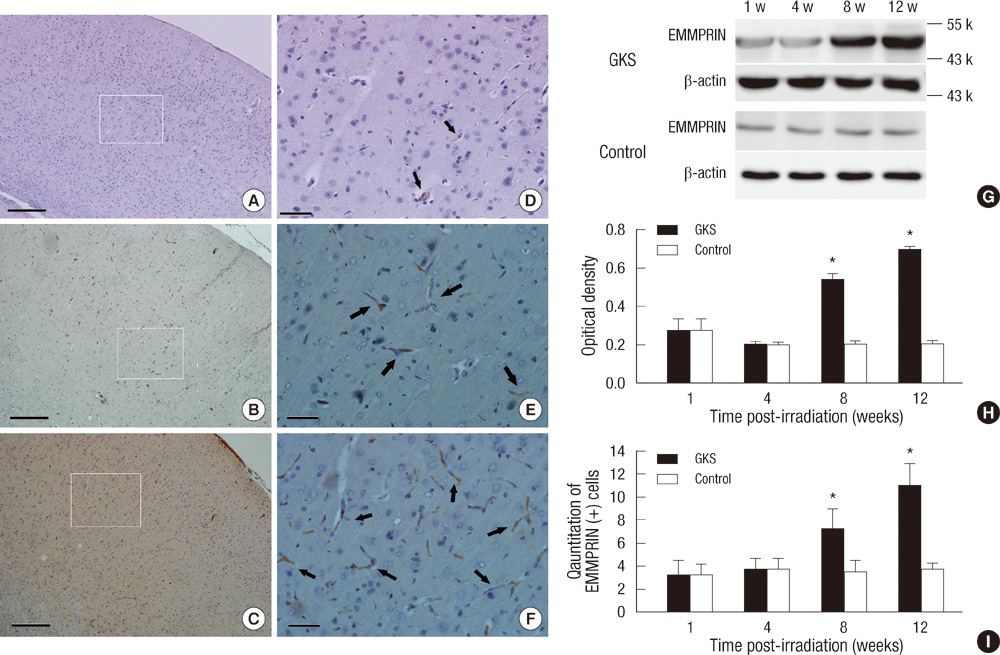
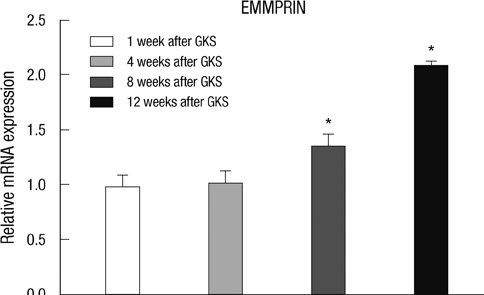
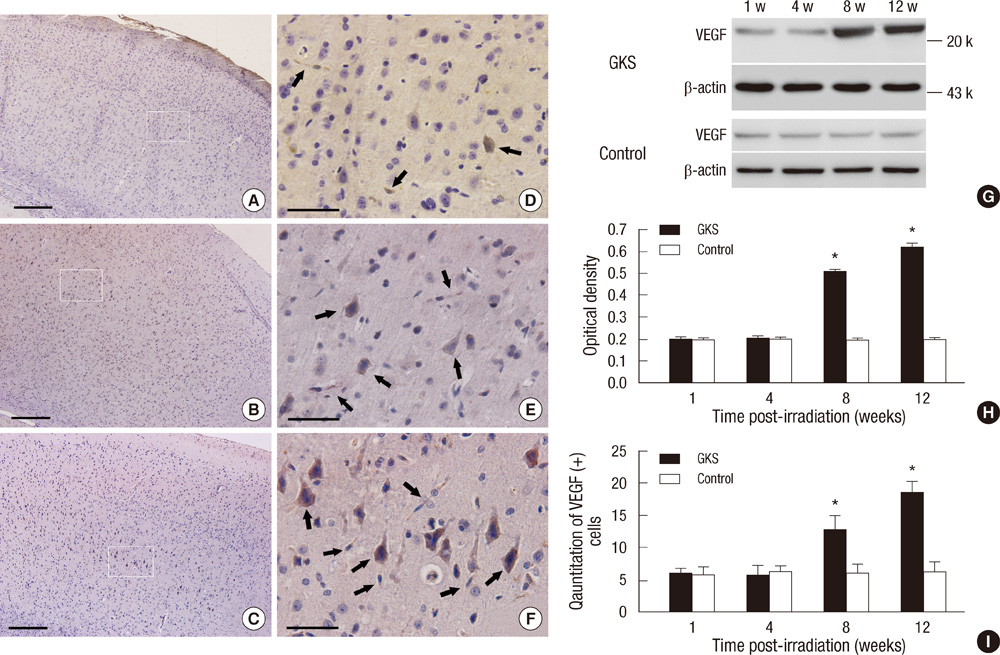
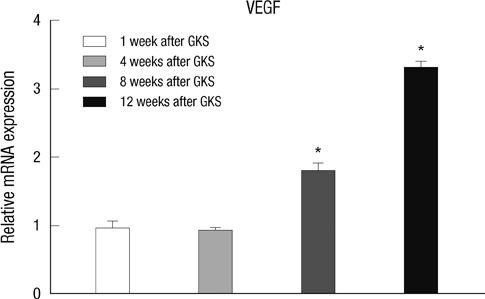
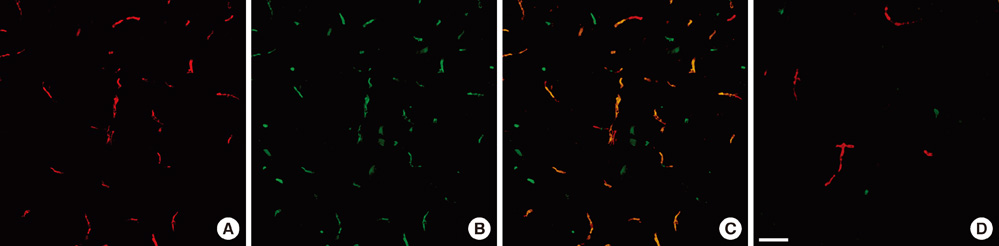
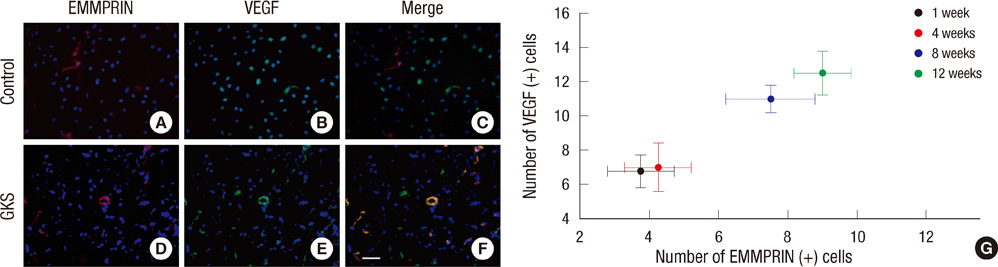
- The extracellular matrix metalloproteinase inducer (EMMPRIN) has been known to play a key regulatory role in pathological angiogenesis. A elevated activation of vascular endothelial growth factor (VEGF) following radiation injury has been shown to mediate blood-brain barrier (BBB) breakdown. However, the roles of EMMPRIN and VEGF in radiation-induced brain injury after gamma knife surgery (GKS) are not clearly understood. In this study, we investigated EMMPRIN changes in a rat model of radiation injury following GKS and examined potential associations between EMMPRIN and VEGF expression. Adult male rats were subjected to cerebral radiation injury by GKS under anesthesia. We found that EMMPRIN and VEGF expression were markedly upregulated in the target area at 8-12 weeks after GKS compared with the control group by western blot, immunohistochemistry, and RT-PCR analysis. Immunofluorescent double staining demonstrated that EMMPRIN signals colocalized with caspase-3 and VEGF-positive cells. Our data also demonstrated that increased EMMPRIN expression was correlated with increased VEGF levels in a temporal manner. This is the first study to show that EMMPRIN and VEGF may play a role in radiation injuries of the central nervous system after GKS.
Keyword
MeSH Terms
-
Animals
Antigens, CD147/*metabolism
Brain/blood supply/metabolism/pathology/*radiation effects
Brain Injuries/metabolism/pathology
Caspase 3/metabolism
Gamma Rays/*adverse effects
Immunohistochemistry
Male
Microscopy, Electron, Transmission
Parietal Lobe/metabolism/pathology/radiation effects
Radiation Injuries, Experimental/metabolism
Radiosurgery/adverse effects
Rats
Rats, Wistar
Time Factors
Vascular Endothelial Growth Factor A/*metabolism
Figure
Reference
-
1. Buis DR, Meijer OW, van den Berg R, Lagerwaard FJ, Bot JC, Slotman BJ, Vandertop WP. Clinical outcome after repeated radiosurgery for brain arteriovenous malformations. Radiother Oncol. 2010. 95:250–256.2. Dewan S, Norén G. Retreatment of vestibular schwannomas with Gamma Knife surgery. J Neurosurg. 2008. 109:144–148.3. Yuan H, Gaber MW, McColgan T, Naimark MD, Kiani MF, Merchant TE. Radiation-induced permeability and leukocyte adhesion in the rat blood-brain barrier: modulation with anti-ICAM-1 antibodies. Brain Res. 2003. 969:59–69.4. Gabison EE, Hoang-Xuan T, Mauviel A, Menashi S. EMMPRIN/CD147, an MMP modulator in cancer, development and tissue repair. Biochimie. 2005. 87:361–368.5. Tang Y, Kesavan P, Nakada MT, Yan L. Tumor-stroma interaction: positive feedback regulation of extracellular matrix metalloproteinase inducer (EMMPRIN) expression and matrix metalloproteinase-dependent generation of soluble EMMPRIN. Mol Cancer Res. 2004. 2:73–80.6. Kato N, Yuzawa Y, Kosugi T, Hobo A, Sato W, Miwa Y, Sakamoto K, Matsuo S, Kadomatsu K. The E-selectin ligand basigin/CD147 is responsible for neutrophil recruitment in renal ischemia/reperfusion. J Am Soc Nephrol. 2009. 20:1565–1576.7. Seizer P, Ochmann C, Schönberger T, Zach S, Rose M, Borst O, Klingel K, Kandolf R, Macdonald HR, Nowak RA, Engelhardt S, Lang F, Gawaz M, May AE. Disrupting the EMMPRIN (CD147)-cyclophilin A interaction reduces infarct size and preserves systolic function after myocardial ischemia and reperfusion. Arterioscler Thromb Vasc Biol. 2011. 31:1377–1386.8. Zhu W, Khachi S, Hao Q, Shen F, Young WL, Yang GY, Chen Y. Upregulation of EMMPRIN after permanent focal cerebral ischemia. Neurochem Int. 2008. 52:1086–1091.9. Nahalkova J, Volkmann I, Aoki M, Winblad B, Bogdanovic N, Tjernberg LO, Behbahani H. CD147, a gamma-secretase associated protein is upregulated in Alzheimer’s disease brain and its cellular trafficking is affected by presenilin-2. Neurochem Int. 2010. 56:67–76.10. Agrawal SM, Silva C, Tourtellotte WW, Yong VW. EMMPRIN: a novel regulator of leukocyte transmigration into the CNS in multiple sclerosis and experimental autoimmune encephalomyelitis. J Neurosci. 2011. 31:669–677.11. Menashi S, Serova M, Ma L, Vignot S, Mourah S, Calvo F. Regulation of extracellular matrix metalloproteinase inducer and matrix metalloproteinase expression by amphiregulin in transformed human breast epithelial cells. Cancer Res. 2003. 63:7575–7580.12. Gabison EE, Mourah S, Steinfels E, Yan L, Hoang-Xuan T, Watsky MA, De Wever B, Calvo F, Mauviel A, Menashi S. Differential expression of extracellular matrix metalloproteinase inducer (CD147) in normal and ulcerated corneas: role in epithelio-stromal interactions and matrix metalloproteinase induction. Am J Pathol. 2005. 166:209–219.13. Dent P, Yacoub A, Contessa J, Caron R, Amorino G, Valerie K, Hagan MP, Grant S, Schmidt-Ullrich R. Stress and radiation-induced activation of multiple intracellular signaling pathways. Radiat Res. 2003. 159:283–300.14. Criscuolo GR. The genesis of peritumoral vasogenic brain edema and tumor cysts: a hypothetical role for tumor-derived vascular permeability factor. Yale J Biol Med. 1993. 66:277–314.15. Zhang ZG, Zhang L, Jiang Q, Zhang R, Davies K, Powers C, Bruggen N, Chopp M. VEGF enhances angiogenesis and promotes blood-brain barrier leakage in the ischemic brain. J Clin Invest. 2000. 106:829–838.16. Li YQ, Ballinger JR, Nordal RA, Su ZF, Wong CS. Hypoxia in radiation-induced blood-spinal cord barrier breakdown. Cancer Res. 2001. 61:3348–3354.17. Kamiryo T, Kassell NF, Thai QA, Lopes MB, Lee KS, Steiner L. Histological changes in the normal rat brain after gamma irradiation. Acta Neurochir (Wien). 1996. 138:451–459.18. Fedak PW, de Sa MP, Verma S, Nili N, Kazemian P, Butany J, Strauss BH, Weisel RD, David TE. Vascular matrix remodeling in patients with bicuspid aortic valve malformations: implications for aortic dilatation. J Thorac Cardiovasc Surg. 2003. 126:797–806.19. Chen Y, Zhang H, Gou X, Horikawa Y, Xing J, Chen Z. Upregulation of HAb18G/CD147 in activated human umbilical vein endothelial cells enhances the angiogenesis. Cancer Lett. 2009. 278:113–121.20. Seulberger H, Unger CM, Risau W. HT7, neurothelin, basigin, gp42 and OX-47: many names for one developmentally regulated immuno-globulin-like surface glycoprotein on blood-brain barrier endothelium, epithelial tissue barriers and neurons. Neurosci Lett. 1992. 140:93–97.21. Burggraf D, Liebetrau M, Martens HK, Wunderlich N, Jäger G, Dichgans M, Hamann GF. Matrix metalloproteinase induction by EMMPRIN in experimental focal cerebral ischemia. Eur J Neurosci. 2005. 22:273–277.22. Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005. 65:3193–3199.23. Huet E, Gabison EE, Mourah S, Menashi S. Role of emmprin/CD147 in tissue remodeling. Connect Tissue Res. 2008. 49:175–179.24. Quemener C, Gabison EE, Naïmi B, Lescaille G, Bougatef F, Podgorniak MP, Labarchède G, Lebbé C, Calvo F, Menashi S, Mourah S. Extracellular matrix metalloproteinase inducer up-regulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res. 2007. 67:9–15.25. Lee SR, Lo EH. Induction of caspase-mediated cell death by matrix metalloproteinases in cerebral endothelial cells after hypoxia-reoxygenation. J Cereb Blood Flow Metab. 2004. 24:720–727.26. Gasche Y, Copin JC, Sugawara T, Fujimura M, Chan PH. Matrix metalloproteinase inhibition prevents oxidative stress-associated blood-brain barrier disruption after transient focal cerebral ischemia. J Cereb Blood Flow Metab. 2001. 21:1393–1400.27. Tsao MN, Li YQ, Lu G, Xu Y, Wong CS. Upregulation of vascular endothelial growth factor is associated with radiation-induced blood-spinal cord barrier breakdown. J Neuropathol Exp Neurol. 1999. 58:1051–1060.28. Zhou Q, Zhu Y, Deng Z, Long H, Zhang S, Chen X. VEGF and EMMPRIN expression correlates with survival of patients with osteosarcoma. Surg Oncol. 2011. 20:13–19.29. Tang Y, Nakada MT, Kesavan P, McCabe F, Millar H, Rafferty P, Bugelski P, Yan L. Extracellular matrix metalloproteinase inducer stimulates tumor angiogenesis by elevating vascular endothelial cell growth factor and matrix metalloproteinases. Cancer Res. 2005. 65:3193–3199.30. Bougatef F, Quemener C, Kellouche S, Naïmi B, Podgorniak MP, Millot G, Gabison EE, Calvo F, Dosquet C, Lebbé C, Menashi S, Mourah S. EMMPRIN promotes angiogenesis through hypoxia-inducible factor-2alpha-mediated regulation of soluble VEGF isoforms and their receptor VEGFR-2. Blood. 2009. 114:5547–5556.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Upregulation of VEGF and FGF2 in Normal Rat Brain after Experimental Intraoperative Radiation Therapy
- Knockdown of EMMPRIN (OX47) in MRMT-1 Carcinoma Cells Inhibits Tumor Growth and Decreases Cancer-Induced Bone Destruction and Pain
- Extracellular Matrix Metalloproteinase Inducer is Regulated Developmentally and Functionally in the Rat Submandibular Gland
- Hyperacute Radiation Effect on Cerebral Cortex after Local Gamma-irradiation in the Rat Brain
- Effect of irradiation on expression of clusterin in the rat salivary glands