J Korean Med Sci.
2012 Jan;27(1):27-35. 10.3346/jkms.2012.27.1.27.
LPS-Induced Migration of Peritoneal B-1 Cells is Associated with Upregulation of CXCR4 and Increased Migratory Sensitivity to CXCL12
- Affiliations
-
- 1Division of Pathology, Department of Molecular Cell Biology and Samsung Biomedical Research Institute, Sungkyunkwan University School of Medicine, Suwon, Korea. tjkim@skku.edu
- KMID: 1792975
- DOI: http://doi.org/10.3346/jkms.2012.27.1.27
Abstract
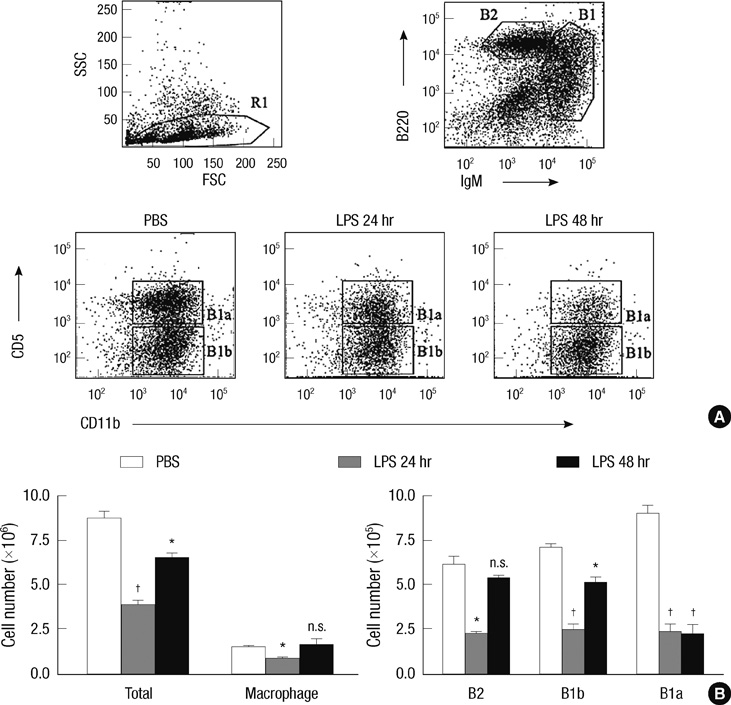
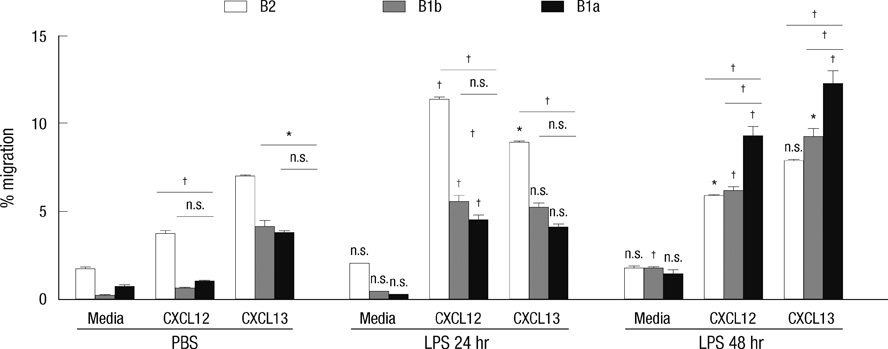
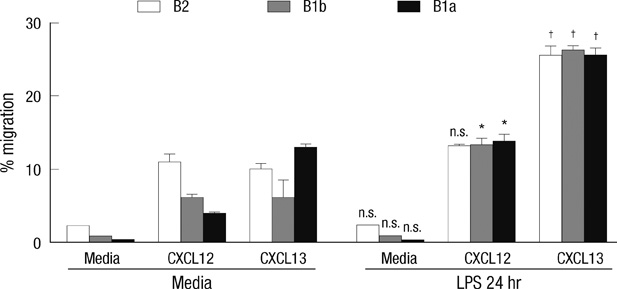
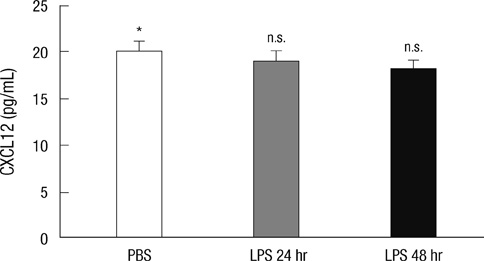
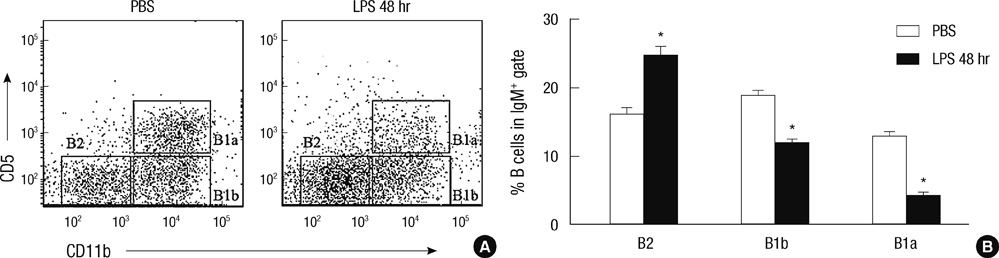
- B-1 cells, which constitute a predominant lymphocyte subset in serosal cavities and produce most of natural antibodies, are subdivided into the CD5+ B-1a and CD5- B-1b cell subpopulations, but the differential roles of B-1a and B-1b cells are not well understood. We report that B-1a cells preferentially migrate out of the peritoneal cavity and upregulate the expression of CXCR4 with heightened sensitivity to CXCL12 and CXCL13 upon LPS treatment compared to B-1b and B-2 cells. Whereas B-1a cells were slightly more abundant than B-1b and B-2 cells in the homeostatic condition, the number of B-1a cells preferentially decreased 48 hr after LPS treatment. The decrease in the peritoneal B-1a cell number was accompanied with increased migration of B-1a cells toward CXCL-12 and CXCL-13 in in vitro transmigration assay using peritoneal B cells from LPS treated mice. The expression level of CXCR4, but not of CXCR5, was also more prominently increased in B-1a cells upon LPS stimulation. LPS-stimulated B-1a cells did not accumulate in omental milky spots in contrast to B-2 cells. These results suggest that B-1a cells actively migrate out of the peritoneal cavity through the regulation of the migratory responsiveness to chemokines and actively participate in systemic immune responses.
Keyword
MeSH Terms
-
Adjuvants, Immunologic/pharmacology
Animals
B-Lymphocytes/cytology/*drug effects/immunology
Cell Movement
Cells, Cultured
Chemokine CXCL12/metabolism/*pharmacology
Chemokine CXCL13/metabolism/pharmacology
Lipopolysaccharides/*pharmacology
Mice
Mice, Inbred C57BL
Peritoneal Cavity/cytology
Receptors, CXCR4/*metabolism
Up-Regulation
Figure
Cited by 2 articles
-
Expansion and Sub-Classification of T Cell-Dependent Antibody Responses to Encompass the Role of Innate-Like T Cells in Antibody Responses
Chanho Park, Tae Jin Kim
Immune Netw. 2018;18(5):. doi: 10.4110/in.2018.18.e34.The Role of B Cells in Transplantation Rejection
Tae Jin Kim
J Korean Soc Transplant. 2018;32(1):1-6. doi: 10.4285/jkstn.2018.32.1.1.
Reference
-
1. Montecino-Rodriguez E, Dorshkind K. New perspectives in B-1 B cell development and function. Trends Immunol. 2006. 27:428–433.2. Hardy RR. B-1 B cells: development, selection, natural autoantibody and leukemia. Curr Opin Immunol. 2006. 18:547–555.3. Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006. 7:293–301.4. Alugupalli KR, Leong JM, Woodland RT, Muramatsu M, Honjo T, Gerstein RM. B1b lymphocytes confer T cell-independent long-lasting immunity. Immunity. 2004. 21:379–390.5. Szczepanik M, Akahira-Azuma M, Bryniarski K, Tsuji RF, Kawikova I, Ptak W, Kiener C, Campos RA, Askenase PW. B-1 B cells mediate required early T cell recruitment to elicit protein-induced delayed-type hypersensitivity. J Immunol. 2003. 171:6225–6235.6. Nogueira-Martins MF, Mariano M. B-1 cell participation in T-cell-mediated alloimmune response. Immunobiology. 2010. 215:264–274.7. Wardemann H, Boehm T, Dear N, Carsetti R. B-1a B cells that link the innate and adaptive immune responses are lacking in the absence of the spleen. J Exp Med. 2002. 195:771–780.8. Baggiolini M. Chemokines and leukocyte traffic. Nature. 1998. 392:565–568.9. Sallusto F, Mackay CR. Chemoattractants and their receptors in homeostasis and inflammation. Curr Opin Immunol. 2004. 16:724–731.10. Förster R, Mattis AE, Kremmer E, Wolf E, Brem G, Lipp M. A putative chemokine receptor, BLR1, directs B cell migration to defined lymphoid organs and specific anatomic compartments of the spleen. Cell. 1996. 87:1037–1047.11. Bowman EP, Campbell JJ, Soler D, Dong Z, Manlongat N, Picarella D, Hardy RR, Butcher EC. Developmental switches in chemokine response profiles during B cell differentiation and maturation. J Exp Med. 2000. 191:1303–1318.12. Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996. 382:635–638.13. Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proc Natl Acad Sci U S A. 1998. 95:9448–9453.14. Palmesino E, Moepps B, Gierschik P, Thelen M. Differences in CXCR4-mediated signaling in B cells. Immunobiology. 2006. 211:377–389.15. Foussat A, Balabanian K, Amara A, Bouchet-Delbos L, Durand-Gasselin I, Baleux F, Couderc J, Galanaud P, Emilie D. Production of stromal cell-derived factor 1 by mesothelial cells and effects of this chemokine on peritoneal B lymphocytes. Eur J Immunol. 2001. 31:350–359.16. Yun HJ, Jo DY. Production of stromal cell-derived factor-1 (SDF-1)and expression of CXCR4 in human bone marrow endothelial cells. J Korean Med Sci. 2003. 18:679–685.17. Rangel-Moreno J, Moyron-Quiroz JE, Carragher DM, Kusser K, Hartson L, Moquin A, Randall TD. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009. 30:731–743.18. Ansel KM, Ngo VN, Hyman PL, Luther SA, Förster R, Sedgwick JD, Browning JL, Lipp M, Cyster JG. A chemokine-driven positive feedback loop organizes lymphoid follicles. Nature. 2000. 406:309–314.19. Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004. 5:943–952.20. Caron G, Le Gallou S, Lamy T, Tarte K, Fest T. CXCR4 expression functionally discriminates centroblasts versus centrocytes within human germinal center B cells. J Immunol. 2009. 182:7595–7602.21. Ansel KM, Harris RB, Cyster JG. CXCL13 is required for B1 cell homing, natural antibody production, and body cavity immunity. Immunity. 2002. 16:67–76.22. Le Y, Zhu BM, Harley B, Park SY, Kobayashi T, Manis JP, Luo HR, Yoshimura A, Hennighausen L, Silberstein LE. SOCS3 protein developmentally regulates the chemokine receptor CXCR4-FAK signaling pathway during B lymphopoiesis. Immunity. 2007. 27:811–823.23. Nakashima H, Hamaguchi Y, Watanabe R, Ishiura N, Kuwano Y, Okochi H, Takahashi Y, Tamaki K, Sato S, Tedder TF, Fujimoto M. CD22 expression mediates the regulatory functions of peritoneal B-1a cells during the remission phase of contact hypersensitivity reactions. J Immunol. 2010. 184:4637–4645.24. Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006. 25:977–988.25. Tarlinton D, Radbruch A, Hiepe F, Dörner T. Plasma cell differentiation and survival. Curr Opin Immunol. 2008. 20:162–169.26. Glodek AM, Honczarenko M, Le Y, Campbell JJ, Silberstein LE. Sustained activation of cell adhesion is a differentially regulated process in B lymphopoiesis. J Exp Med. 2003. 197:461–473.27. Baumgarth N. The double life of a B-1 cell: self-reactivity selects for protective effector functions. Nat Rev Immunol. 2011. 11:34–46.28. Chumley MJ, Dal Porto JM, Cambier JC. The unique antigen receptor signaling phenotype of B-1 cells is influenced by locale but induced by antigen. J Immunol. 2002. 169:1735–1743.29. Krist LF, Eestermans IL, Steenbergen JJ, Hoefsmit EC, Cuesta MA, Meyer S, Beelen RH. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995. 241:163–174.30. Carlow DA, Gold MR, Ziltener HJ. Lymphocytes in the peritoneum home to the omentum and are activated by resident dendritic cells. J Immunol. 2009. 183:1155–1165.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Extracellular Hsp70 Is Involved in the CXCL12/CXCR4 Pathway in Primary Human Nasal Epithelial Cells: A Preliminary Study
- Targeting the CXCL12/CXCR4 axis in acute myeloid leukemia: from bench to bedside
- Isoorientin Suppresses Invasion of Breast and Colon Cancer Cells by Inhibition of CXC Chemokine Receptor 4 Expression
- The Role of SDF-1α-CXCR4/CXCR7 in Migration of Human Periodontal Ligament Stem Cells
- Expression and functional roles of the chemokine receptor CXCR7 in acute myeloid leukemia cells