J Korean Med Sci.
2013 Apr;28(4):542-549. 10.3346/jkms.2013.28.4.542.
Immunophenotypic Characterization and Quantification of Neoplastic Bone Marrow Plasma Cells by Multiparametric Flow Cytometry and Its Clinical Significance in Korean Myeloma Patients
- Affiliations
-
- 1Department of Laboratory Medicine, University of Ulsan, College of Medicine and Asan Medical Center, Seoul, Korea. cjpark@amc.seoul.kr
- 2Department of Internal Medicine, University of Ulsan, College of Medicine and Asan Medical Center, Seoul, Korea.
- KMID: 1786961
- DOI: http://doi.org/10.3346/jkms.2013.28.4.542
Abstract
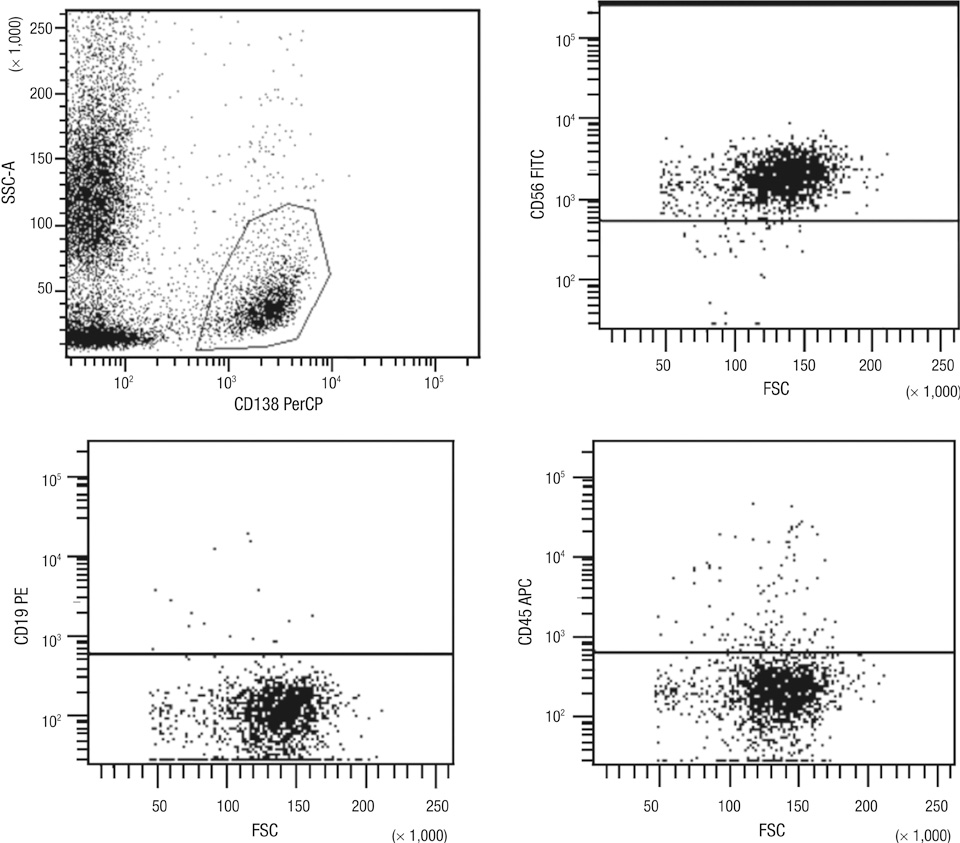
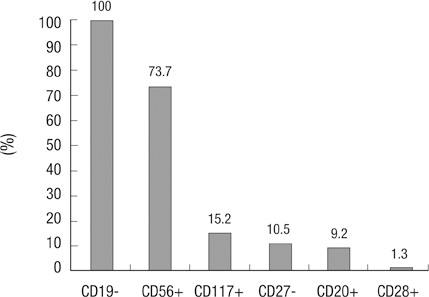
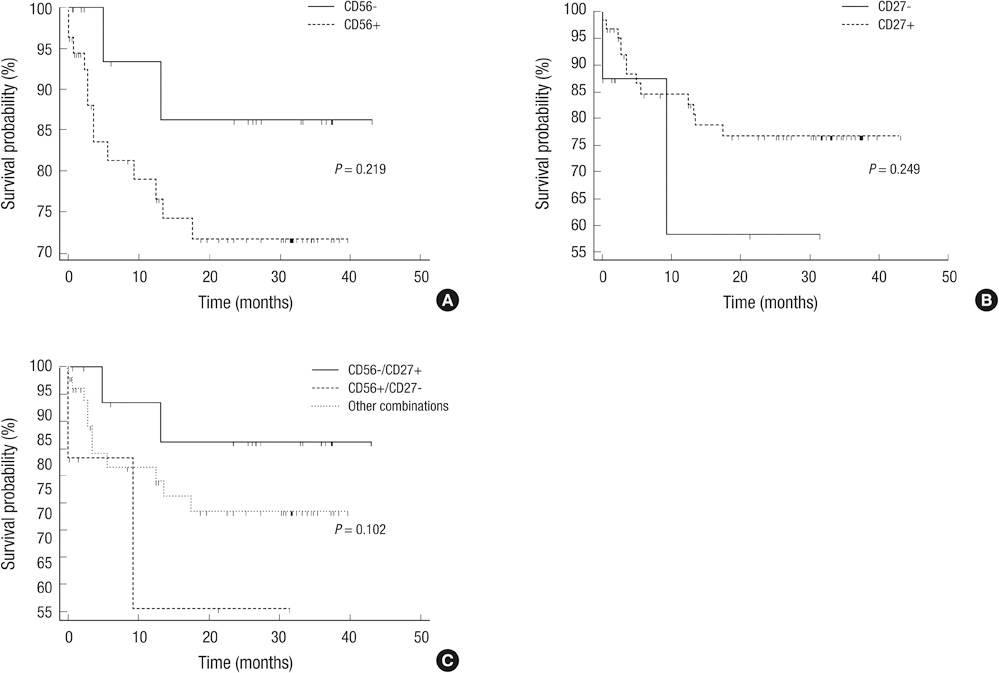
- Multiparametric flow cytometry (MFC) allows discrimination between normal and neoplastic plasma cells (NeoPCs) within the bone marrow plasma cell (BMPC) compartment. This study sought to characterize immunophenotypes and quantitate the proportion of NeoPCs in BMPCs to diagnose plasma cell myeoma (PCM) and evaluate the prognostic impact of this method. We analyzed the MFC data of the bone marrow aspirates of 76 patients with PCM and 33 patients with reactive plasmacytosis. MFC analysis was performed using three combinations: CD38/CD138/-/CD45; CD56/CD20/CD138/CD19; and CD27/CD28/CD138/CD117. The plasma cells of patients with reactive plasmacytosis demonstrated normal immunophenotypic patterns. Aberrant marker expression was observed in NeoPCs, with negative CD19 expression observed in 100% of cases, CD56+ in 73.7%, CD117+ in 15.2%, CD27- in 10.5%, CD20+ in 9.2%, and CD28+ in 1.3%. In PCM patients, more than 20% of NeoPCs/BMPCs were significantly associated with factors suggestive of poor clinical outcomes. Patients who were CD27- or CD56+/CD27-, demonstrated shorter overall survival than patients of other CD56/CD27 combinations. Our results support the clinical value of immunophenotyping and quantifying NeoPCs in PCM patients. This strategy could help to reveal poor prognostic categories and delineate surrogate markers for risk stratification in PCM patients.
MeSH Terms
-
Adult
Aged
Aged, 80 and over
Antigens, CD27/metabolism
Antigens, CD56/metabolism
Asian Continental Ancestry Group
Bone Marrow Cells/*cytology/metabolism
Female
Flow Cytometry
Humans
*Immunophenotyping
Kaplan-Meier Estimate
Male
Middle Aged
Multiple Myeloma/metabolism/mortality/*pathology
Neoplasm Staging
Neoplastic Stem Cells/*cytology/metabolism
Prognosis
Republic of Korea
Risk Factors
Antigens, CD27
Antigens, CD56
Figure
Reference
-
1. Kumar S, Kimlinger T, Morice W. Immunophenotyping in multiple myeloma and related plasma cell disorders. Best Pract Res Clin Haematol. 2010. 23:433–451.2. Paiva B, Almeida J, Pérez-Andrés M, Mateo G, López A, Rasillo A, Vídriales MB, López-Berges MC, Miguel JF, Orfao A. Utility of flow cytometry immunophenotyping in multiple myeloma and other clonal plasma cell-related disorders. Cytometry B Clin Cytom. 2010. 78:239–252.3. Raja KR, Kovarova L, Hajek R. Review of phenotypic markers used in flow cytometric analysis of MGUS and MM, and applicability of flow cytometry in other plasma cell disorders. Br J Haematol. 2010. 149:334–351.4. San Miguel JF, Almeida J, Mateo G, Bladé J, López-Berges C, Caballero D, Hernández J, Moro MJ, Fernández-Calvo J, Díaz-Mediavilla J, Palomera L, Orfao A. Immunophenotypic evaluation of the plasma cell compartment in multiple myeloma: a tool for comparing the efficacy of different treatment strategies and predicting outcome. Blood. 2002. 99:1853–1856.5. Lin P, Owens R, Tricot G, Wilson CS. Flow cytometric immunophenotypic analysis of 306 cases of multiple myeloma. Am J Clin Pathol. 2004. 121:482–488.6. Pérez-Persona E, Vidriales MB, Mateo G, García-Sanz R, Mateos MV, de Coca AG, Galende J, Martín-Nuñez G, Alonso JM, de Las Heras N, et al. New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood. 2007. 110:2586–2592.7. Paiva B, Vidriales MB, Cerveró J, Mateo G, Pérez JJ, Montalbán MA, Sureda A, Montejano L, Gutiérrez NC, García de Coca A, et al. Multiparameter flow cytometric remission is the most relevant prognostic factor for multiple myeloma patients who undergo autologous stem cell transplantation. Blood. 2008. 112:4017–4023.8. Mateo G, Montalbán MA, Vidriales MB, Lahuerta JJ, Mateos MV, Gutiérrez N, Rosiñol L, Montejano L, Bladé J, Martínez R, et al. Prognostic value of immunophenotyping in multiple myeloma: a study by the PETHEMA/GEM cooperative study groups on patients uniformly treated with high-dose therapy. J Clin Oncol. 2008. 26:2737–2744.9. Olteanu H, Wang HY, Chen W, McKenna RW, Karandikar NJ. Immunophenotypic studies of monoclonal gammopathy of undetermined significance. BMC Clin Pathol. 2008. 8:13.10. Gupta R, Bhaskar A, Kumar L, Sharma A, Jain P. Flow cytometric immunophenotyping and minimal residual disease analysis in multiple myeloma. Am J Clin Pathol. 2009. 132:728–732.11. Paiva B, Vidriales MB, Pérez JJ, Mateo G, Montalbán MA, Mateos MV, Bladé J, Lahuerta JJ, Orfao A, San Miguel JF. Multiparameter flow cytometry quantification of bone marrow plasma cells at diagnosis provides more prognostic information than morphological assessment in myeloma patients. Haematologica. 2009. 94:1599–1602.12. Paiva B, Vidriales MB, Mateo G, Pérez JJ, Montalbán MA, Sureda A, Montejano L, Gutiérrez NC, García de Coca A, de las Heras N, et al. The persistence of immunophenotypically normal residual bone marrow plasma cells at diagnosis identifies a good prognostic subgroup of symptomatic multiple myeloma patients. Blood. 2009. 114:4369–4372.13. Johnsen HE, Bøgsted M, Klausen TW, Gimsing P, Schmitz A, Kjaersgaard E, Damgaard T, Voss P, Knudsen LM, Mylin AK, et al. Multiparametric flow cytometry profiling of neoplastic plasma cells in multiple myeloma. Cytometry B Clin Cytom. 2010. 78:338–347.14. Cannizzo E, Bellio E, Sohani AR, Hasserjian RP, Ferry JA, Dorn ME, Sadowski C, Bucci JJ, Carulli G, Preffer F. Multiparameter immunophenotyping by flow cytometry in multiple myeloma: the diagnostic utility of defining ranges of normal antigenic expression in comparison to histology. Cytometry B Clin Cytom. 2010. 78:231–238.15. Frébet E, Abraham J, Geneviève F, Lepelley P, Daliphard S, Bardet V, Amsellem S, Guy J, Mullier F, Durrieu F, et al. A GEIL flow cytometry consensus proposal for quantification of plasma cells: application to differential diagnosis between MGUS and myeloma. Cytometry B Clin Cytom. 2011. 80:176–185.16. Rawstron AC, Orfao A, Beksac M, Bezdickova L, Brooimans RA, Bumbea H, Dalva K, Fuhler G, Gratama J, Hose D, et al. Report of the European Myeloma Network on multiparametric flow cytometry in multiple myeloma and related disorders. Haematologica. 2008. 93:431–438.17. Kyle RA, Rajkumar SV. Criteria for diagnosis, staging, risk stratification and response assessment of multiple myeloma. Leukemia. 2009. 23:3–9.18. Munshi NC, Anderson KC, Bergsagel PL, Shaughnessy J, Palumbo A, Durie B, Fonseca R, Stewart AK, Harousseau JL, Dimopoulos M, et al. Consensus recommendations for risk stratification in multiple myeloma: report of the International Myeloma Workshop Consensus Panel 2. Blood. 2011. 117:4696–4700.19. Sahara N, Takeshita A, Shigeno K, Fujisawa S, Takeshita K, Naito K, Ihara M, Ono T, Tamashima S, Nara K, et al. Clinicopathological and prognostic characteristics of CD56-negative multiple myeloma. Br J Haematol. 2002. 117:882–885.20. Hundemer M, Klein U, Hose D, Raab MS, Cremer FW, Jauch A, Benner A, Heiss C, Moos M, Ho AD, et al. Lack of CD56 expression on myeloma cells is not a marker for poor prognosis in patients treated by high-dose chemotherapy and is associated with translocation t(11;14). Bone Marrow Transplant. 2007. 40:1033–1037.21. Guikema JE, Hovenga S, Vellenga E, Conradie JJ, Abdulahad WH, Bekkema R, Smit JW, Zhan F, Shaughnessy J Jr, Bos NA. CD27 is heterogeneously expressed in multiple myeloma: low CD27 expression in patients with high-risk disease. Br J Haematol. 2003. 121:36–43.22. Moreau P, Robillard N, Jégo G, Pellat C, Le Gouill S, Thoumi S, Avet-Loiseau H, Harousseau JL, Bataille R. Lack of CD27 in myeloma delineates different presentation and outcome. Br J Haematol. 2006. 132:168–170.23. Park HJ, Park EH, Jung KW, Kong HJ, Won YJ, Lee JY, Yoon JH, Park BK, Lee H, Eom HS, et al. Statistics of hematologic malignancies in Korea: incidence, prevalence and survival rates from 1999 to 2008. Korean J Hematol. 2012. 47:28–38.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Simplified flow cytometric immunophenotyping panel for multiple myeloma, CD56/CD19/CD138(CD38)/CD45, to differentiate neoplastic myeloma cells from reactive plasma cells
- Utility of Plasma Cell Screening Tube Kit to Differentiate Neoplastic Plasma Cells from Reactive Plasma Cells
- The Increased Expression and Diagnostic Usefulness of CD56 Antigen in Paraffin Embedded Plasma Cell Neoplasm
- SOLITARY PLASMA CELL MYELOMA ON ANTERIOR MAXILLA: A CASE REPORT
- A Case of Multiple Myeloma in Bilateral Paranasal Sinuses with Loss of Vision