J Korean Med Sci.
2013 Mar;28(3):466-471. 10.3346/jkms.2013.28.3.466.
Antifactor Xa Levels in Critically Ill Korean Patients Receiving Enoxaparin for Thromboprophylaxis: A Prospective Observational Study
- Affiliations
-
- 1Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. suhgy@skku.edu
- 2Department of Laboratory Medicine & Genetics, Seoul, Korea.
- 3Medical Intensive Care Unit, Samsung Medical Center, Seoul, Korea.
- 4Division of Pulmonary and Critical Care Medicine, Department of Medicine, Samsung Changwon Hospital, Sungkyunkwan University School of Medicine, Changwon, Korea.
- 5Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea.
- KMID: 1786947
- DOI: http://doi.org/10.3346/jkms.2013.28.3.466
Abstract
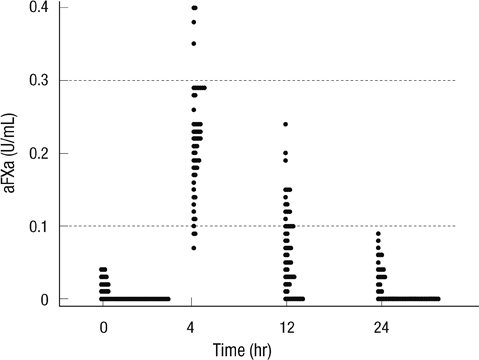
- The aim of this study was to investigate antifactor Xa (aFXa) levels after once daily dose of 40 mg of enoxaparin and to evaluate factors influencing aFXa levels among Korean intensive care unit (ICU) patients. This prospective observational study was conducted between August and December 2011 in medical ICUs at Samsung Medical Center. AFXa levels between 0.1 and 0.3 U/mL were considered to be effective for antithrombotic activity. Fifty-five patients were included. The median aFXa levels were 0.22 (IQR 0.17-0.26) at 4 hr, 0.06 (IQR 0.02-0.1) at 12 hr, and 0 U/mL (IQR 0-0.03) at 24 hr. The numbers of patients showing effective antithrombotic aFXa levels were 48 (87.3%), 18 (32.7%), and 0 (0%) at 4, 12 and 24 hr, respectively. At 12 hr, higher sequential organ failure assessment (SOFA) scores and hyperbilirubinemia were significantly associated with low aFXa levels (OR, 0.58; 95% CI, 0.36-0.93; P = 0.02 and 0.06; 0.003-0.87; 0.04, respectively). Once daily dose of 40 mg of enoxaparin is inadequate for maintaining effective antithrombotic aFXa levels, and the inadequacy is more salient for patients with high SOFA scores and hyperbilirubinemia.
Keyword
MeSH Terms
-
Aged
Asian Continental Ancestry Group
Critical Illness
Enoxaparin/*therapeutic use
Factor Xa/analysis/*antagonists & inhibitors
Female
Fibrinolytic Agents/*therapeutic use
Humans
Hyperbilirubinemia/metabolism
Intensive Care Units
Male
Middle Aged
Odds Ratio
Prospective Studies
Regression Analysis
Republic of Korea
Risk Factors
Venous Thromboembolism/*drug therapy
Enoxaparin
Fibrinolytic Agents
Factor Xa
Figure
Reference
-
1. Kahn SR, Lim W, Dunn AS, Cushman M, Dentali F, Akl EA, Cook DJ, Balekian AA, Klein RC, Le H, et al. Prevention of VTE in nonsurgical patients: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012. 141:e195S–e226S.2. Cook DJ, Crowther MA, Meade MO, Douketis J. VTE in the ICU Workshop Participants. Prevalence, incidence, and risk factors for venous thromboembolism in medical-surgical intensive care unit patients. J Crit Care. 2005. 20:309–313.3. Bang SM, Jang MJ, Oh D, Kim YK, Kim IH, Yoon SS, Yoon HJ, Kim CS, Park S. Korean guidelines for the prevention of venous thromboembolism. J Korean Med Sci. 2010. 25:1553–1559.4. Mayr AJ, Dünser M, Jochberger S, Fries D, Klingler A, Joannidis M, Hasibeder W, Schobersberger W. Antifactor Xa activity in intensive care patients receiving thromboembolic prophylaxis with standard doses of enoxaparin. Thromb Res. 2002. 105:201–204.5. Robinson S, Zincuk A, Strøm T, Larsen TB, Rasmussen B, Toft P. Enoxaparin, effective dosage for intensive care patients: double-blinded, randomised clinical trial. Crit Care. 2010. 14:R41.6. Bjornsson TD, Wagner JA, Donahue SR, Harper D, Karim A, Khouri MS, Murphy WR, Roman K, Schneck D, Sonnichsen DS, et al. A review and assessment of potential sources of ethnic differences in drug responsiveness. J Clin Pharmacol. 2003. 43:943–967.7. Yu CM, Chan TY, Tsoi WC, Sanderson JE. Heparin therapy in the Chinese: lower doses are required. QJM. 1997. 90:535–543.8. Shimada YJ, Nakra NC, Fox JT, Kanei Y. Relation of race (Asian, African-American, European-American, and Hispanic) to activated clotting time after weight-adjusted bolus of heparin during percutaneous coronary intervention. Am J Cardiol. 2010. 105:629–632.9. Cheng TO. Ethnic differences in incidence of diseases and response to medicines. J Natl Med Assoc. 2003. 95:404–405.10. Radtke KP, Greengard JS, Fernández JA, Villoutreix BO, Griffin JH. A two-allele polymorphism in protein C inhibitor with varying frequencies in different ethnic populations. Thromb Haemost. 1996. 75:62–69.11. Metnitz PG, Moreno RP, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR. SAPS 3: from evaluation of the patient to evaluation of the intensive care unit: part 1: objectives, methods and cohort description. Intensive Care Med. 2005. 31:1336–1344.12. Moreno RP, Metnitz PG, Almeida E, Jordan B, Bauer P, Campos RA, Iapichino G, Edbrooke D, Capuzzo M, Le Gall JR. SAPS 3: from evaluation of the patient to evaluation of the intensive care unit: part 2: development of a prognostic model for hospital mortality at ICU admission. Intensive Care Med. 2005. 31:1345–1355.13. Levine MN, Planes A, Hirsh J, Goodyear M, Vochelle N, Gent M. The relationship between anti-factor Xa level and clinical outcome in patients receiving enoxaparine low molecular weight heparin to prevent deep vein thrombosis after hip replacement. Thromb Haemost. 1989. 62:940–944.14. Darze ES, Latado AL, Guimarães AG, Guedes RA, Santos AB, de Moura SS, Passos LC. Incidence and clinical predictors of pulmonary embolism in severe heart failure patients admitted to a coronary care unit. Chest. 2005. 128:2576–2580.15. Cohen AT, Davidson BL, Gallus AS, Lassen MR, Prins MH, Tomkowski W, Turpie AG, Egberts JF, Lensing AW. ARTEMIS Investigators. Efficacy and safety of fondaparinux for the prevention of venous thromboembolism in older acute medical patients: randomised placebo controlled trial. BMJ. 2006. 332:325–329.16. Lederle FA, Sacks JM, Fiore L, Landefeld CS, Steinberg N, Peters RW, Eid AA, Sebastian J, Stasek JE Jr, Fye CL. The prophylaxis of medical patients for thromboembolism pilot study. Am J Med. 2006. 119:54–59.17. Brophy DF, Bukaveckas BL, Ferreira-Gonzalez A, Archer KJ, Martin EJ, Gehr TW. A pilot study of genetic polymorphisms and hemodialysis vascular access thrombosis. Hemodial Int. 2009. 13:19–26.18. Priglinger U, Delle Karth G, Geppert A, Joukhadar C, Graf S, Berger R, Hülsmann M, Spitzauer S, Pabinger I, Heinz G. Prophylactic anticoagulation with enoxaparin: is the subcutaneous route appropriate in the critically ill? Crit Care Med. 2003. 31:1405–1409.19. Van PY, Cho SD, Underwood SJ, Morris MS, Watters JM, Schreiber MA. Thrombelastography versus AntiFactor Xa levels in the assessment of prophylactic-dose enoxaparin in critically ill patients. J Trauma. 2009. 66:1509–1515.20. Burroughs VJ, Maxey RW, Levy RA. Racial and ethnic differences in response to medicines: towards individualized pharmaceutical treatment. J Natl Med Assoc. 2002. 94:1–26.21. Kim TW, Kim WK, Lee JH, Kim SB, Kim SW, Suh C, Lee KH, Lee JS, Seo EJ, Chi HS, et al. Low prevalence of activated protein C resistance and coagulation factor V Arg506 to Gln mutation among Korean patients with deep vein thrombosis. J Korean Med Sci. 1998. 13:587–590.22. Malinoski D, Jafari F, Ewing T, Ardary C, Conniff H, Baje M, Kong A, Lekawa ME, Dolich MO, Cinat ME, et al. Standard prophylactic enoxaparin dosing leads to inadequate anti-Xa levels and increased deep venous thrombosis rates in critically ill trauma and surgical patients. J Trauma. 2010. 68:874–880.23. Dörffler-Melly J, de Jonge E, Pont AC, Meijers J, Vroom MB, Büller HR, Levi M. Bioavailability of subcutaneous low-molecular-weight heparin to patients on vasopressors. Lancet. 2002. 359:849–850.24. Hutton RA. Chromogenic substrates in haemostasis. Blood Rev. 1987. 1:201–206.25. Kessler CM, Esparraguera IM, Jacobs HM, Druy E, Fortune WP, Holloway DS, Giordano J, Davidson BL. Monitoring the anticoagulant effects of a low molecular weight heparin preparation: correlation of assays in orthopedic surgery patients receiving ardeparin sodium for prophylaxis of deep venous thrombosis. Am J Clin Pathol. 1995. 103:642–648.26. Boneu B. Low molecular weight heparin therapy: is monitoring needed? Thromb Haemost. 1994. 72:330–334.27. Hemker HC, Wielders S, Kessels H, Béguin S. Continuous registration of thrombin generation in plasma, its use for the determination of the thrombin potential. Thromb Haemost. 1993. 70:617–624.28. Vincent PD, Albert M, Champagne MC, Zikos T, Boulanger I, Blais L, Williamson DR. Factors influencing enoxaparin anti-Xa activity in surgical critically ill patients. J Crit Care. 2011. 26:347–351.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Augmented renal clearance
- Pharmacological and Mechanical Thromboprophylaxis in Critically Ill Patients: a Network Meta-Analysis of 12 Trials
- Thromboprophylaxis after bariatric surgery
- Low molecular-weight heparin for thromboprophylaxis in patients undergoing gastric cancer surgery: an experience from one Korean institute
- Enoxaparin-associated Retroperitoneal Hematoma in Two Chronic Dialysis Patients