J Korean Med Sci.
2013 Mar;28(3):450-460. 10.3346/jkms.2013.28.3.450.
Permissive Hyperglycemia in Extremely Low Birth Weight Infants
- Affiliations
-
- 1Department of Pediatrics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea. wonspark@skku.edu
- KMID: 1786945
- DOI: http://doi.org/10.3346/jkms.2013.28.3.450
Abstract
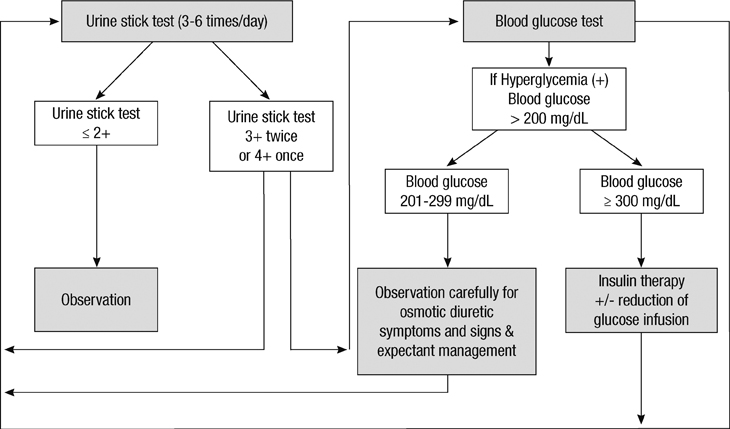
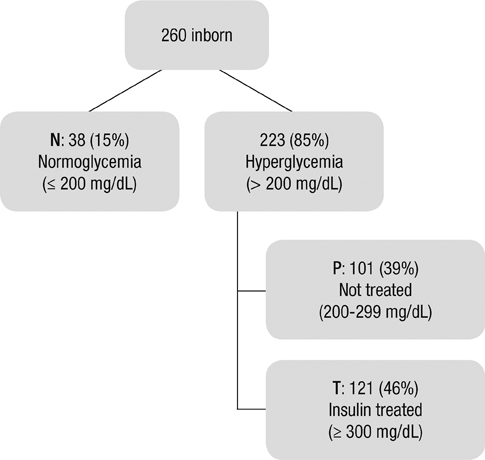
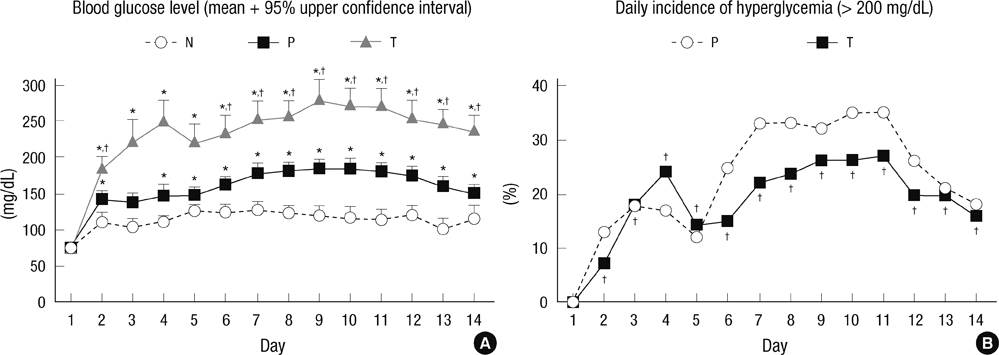
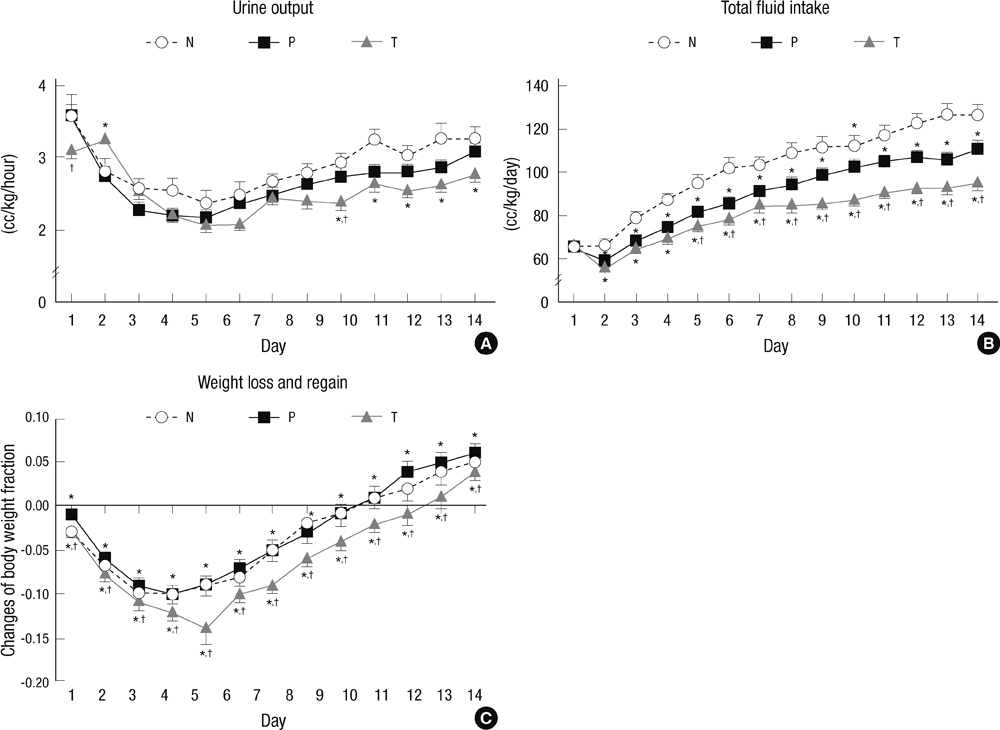
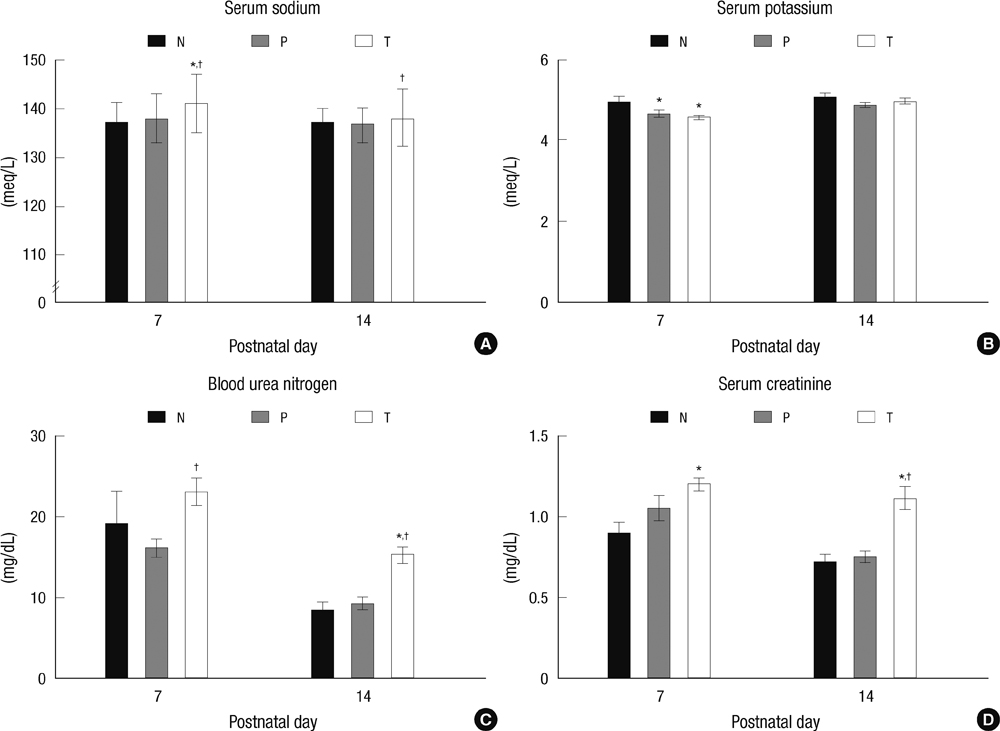
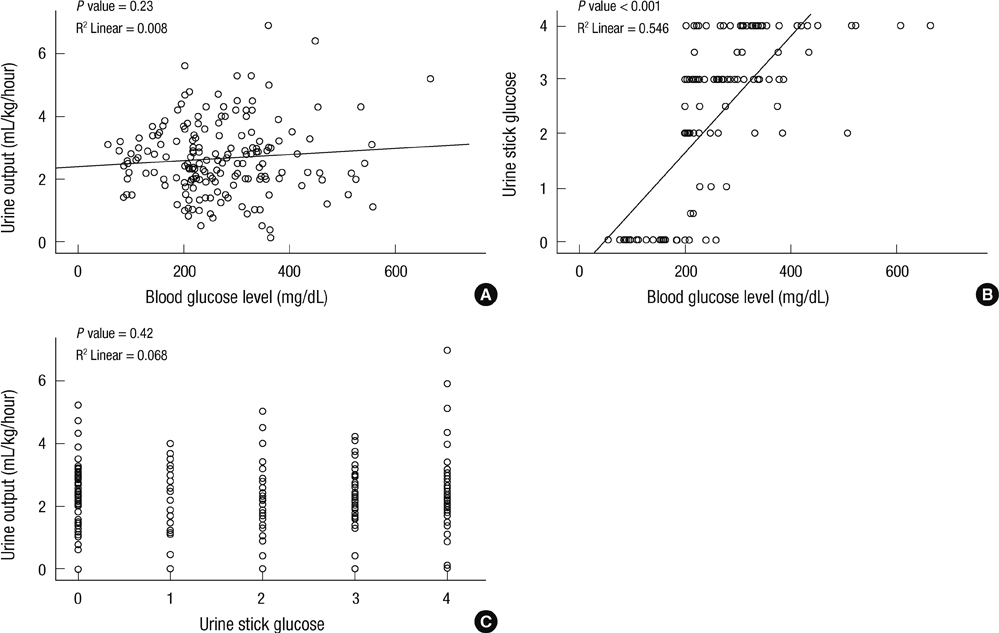
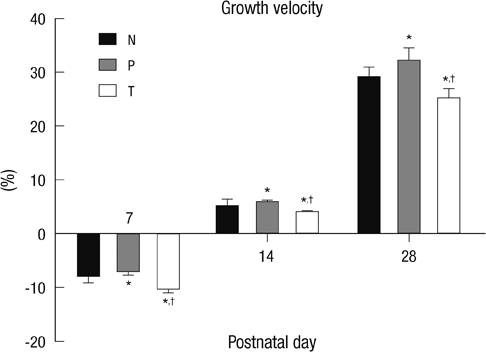
- The aim of this study is to evaluate the outcomes of permissive hyperglycemia up to < 300 mg/dL in extremely-low-birth-weight infants (ELBWIs). We retrospectively reviewed the medical records of 260 live-born ELBWIs at Samsung Medical Center between 2004 and 2008, grouped according to peak blood glucose level and management during the first 14 days of life. The groups were normoglycemia (N), blood glucose < or = 200 mg/dL; permissive hyperglycemia (P), blood glucose 201-299 mg/dL without insulin treatment; treated hyperglycemia (T), blood glucose > or = 300 mg/dL with insulin. Only 15% of patients were grouped as N, with 39% as P and 46% as T. Although P had lower birth weight, P had a similar daily calorie and glucose intake as well as urine output compared to N. There was no significant correlation between blood glucose level and urine output on day 7. Compared to N, P showed faster weight gain and similar mortality, morbidities, and long-term neurological outcomes. Permissive hyperglycemia up to < 300 mg/dL without insulin treatment during the first 14 days of life is not associated with osmotic diuresis or increased mortality or morbidities, suggesting that it is not detrimental in ELBWIs.
MeSH Terms
-
Blood Glucose/analysis
Demography
Gestational Age
Humans
Hyperglycemia/*blood/drug therapy/mortality
Hypoglycemic Agents/therapeutic use
Infant, Extremely Low Birth Weight
Infant, Newborn
Infant, Premature, Diseases/*blood/mortality/pathology
Intensive Care Units, Neonatal
Odds Ratio
Retrospective Studies
Time Factors
Blood Glucose
Hypoglycemic Agents
Figure
Cited by 1 articles
-
Clinical Characteristics of Early Onset Sepsis in Micropreemie Born at 25 or Less than 25 Weeks of Gestational Age
Shin Ae Yoon, Ji Young Chun, Yo Han Ho, Ji Sook Kim, Hye Soo Yoo, Se In Sung, So Yoon Ahn, Yun Sil Chang, Won Soon Park
Korean J Perinatol. 2016;27(1):53-59. doi: 10.14734/kjp.2016.27.1.53.
Reference
-
1. Dweck HS, Cassady G. Glucose intolerance in infants of very low birth weight: I. incidence of hyperglycemia in infants of birth weights 1,100 grams or less. Pediatrics. 1974. 53:189–195.2. Binder ND, Raschko PK, Benda GI, Reynolds JW. Insulin infusion with parenteral nutrition in extremely low birth weight infants with hyperglycemia. J Pediatr. 1989. 114:273–280.3. Hays SP, Smith EO, Sunehag AL. Hyperglycemia is a risk factor for early death and morbidity in extremely low birth-weight infants. Pediatrics. 2006. 118:1811–1818.4. Garg R, Agthe AG, Donohue PK, Lehmann CU. Hyperglycemia and retinopathy of prematurity in very low birth weight infants. J Perinatol. 2003. 23:186–194.5. Decaro MH, Vain NE. Hyperglycaemia in preterm neonates: what to know, what to do. Early Hum Dev. 2011. 87:S19–S22.6. Hay WW Jr. Addressing hypoglycemia and hyperglycemia. Pediatr Rev. 1999. 20:e4–e5.7. Hey E. Hyperglycaemia and the very preterm baby. Semin Fetal Neonatal Med. 2005. 10:377–387.8. Hemachandra AH, Cowett RM. Neonatal hyperglycemia. Pediatr Rev. 1999. 20:e16–e24.9. Kairamkonda VR, Khashu M. Controversies in the management of hyperglycemia in the ELBW infant. Indian Pediatr. 2008. 45:29–38.10. Coulthard MG, Hey EN. Renal processing of glucose in well and sick neonates. Arch Dis Child Fetal Neonatal Ed. 1999. 81:F92–F98.11. Beardsall K, Vanhaesebrouck S, Ogilvy-Stuart AL, Vanhole C, Palmer CR, van Weissenbruch M, Midgley P, Thompson M, Thio M, Cornette L, et al. Early insulin therapy in very-low-birth-weight infants. N Engl J Med. 2008. 359:1873–1884.12. Han BR, Oh YS, Ahn KH, Kim HY, Hong SC, Oh MJ, Kim HJ, Kim YT, Lee KW, Kim SH. Clinical implication of 2nd trimester glycosuria. Korean J Perinatol. 2010. 21:258–265.13. Pati NK, Maheshwari R, Pati NK, Salhan RN. Transient neonatal hyperglycemia. Indian Pediatr. 2001. 38:898–901.14. Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966. 37:403–408.15. Bae CW, Hahn WH. Surfactant therapy for neonatal respiratory distress syndrome: a review of Korean experiences over 17 years. J Korean Med Sci. 2009. 24:1110–1118.16. Ehrenkranz RA, Walsh MC, Vohr BR, Jobe AH, Wright LL, Fanaroff AA, Wrage LA, Poole K. National Institutes of Child Health and Human Development Neonatal Research Network. Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005. 116:1353–1360.17. Choi CW, Kim BI, Kim EK, Song ES, Lee JJ. Incidence of bronchopulmonary dysplasia in Korea. J Korean Med Sci. 2012. 27:914–921.18. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978. 92:529–534.19. Lee JY, Kim HS, Jung E, Kim ES, Shim GH, Lee HJ, Lee JA, Choi CW, Kim EK, Kim BI, et al. Risk factors for periventricular-intraventricular hemorrhage in premature infants. J Korean Med Sci. 2010. 25:418–424.20. Bell MJ, Ternberg JL, Feigin RD, Keating JP, Marshall R, Barton L, Brotherton T. Neonatal necrotizing enterocolitis: therapeutic decisions based upon clinical staging. Ann Surg. 1978. 187:1–7.21. An international classification of retinopathy of prematurity: the Committee for the Classification of Retinopathy of Prematurity. Arch Ophthalmol. 1984. 102:1130–1134.22. Stoll BJ, Hansen NI, Adams-Chapman I, Fanaroff AA, Hintz SR, Vohr B, Higgins RD. National Institute of Child Health and Human Development Neonatal Research Network. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA. 2004. 292:2357–2365.23. Leonard CH, Piecuch RE, Cooper BA. Use of the Bayley Infant Neurodevelopmental Screener with low birth weight infants. J Pediatr Psychol. 2001. 26:33–40.24. Osborn DA, Evans N, Kluckow M. Hemodynamic and antecedent risk factors of early and late periventricular/intraventricular hemorrhage in premature infants. Pediatrics. 2003. 112:33–39.25. Xu FL, Duan JJ, Zhang YH, Zhang XL, Guo JJ. Risk factors for periventricular-intraventricular hemorrhage in premature infants treated with mechanical ventilation. Zhongguo Dang Dai Er Ke Za Zhi. 2012. 14:838–841.26. Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001. 107:270–273.27. Levitsky DA, Strupp BJ. Malnutrition and the brain: changing concepts, changing concerns. J Nutr. 1995. 125:2212S–2220S.28. Grantham-McGregor S. A review of studies of the effect of severe malnutrition on mental development. J Nutr. 1995. 125:2233S–2238S.29. Dobbing J. Davidson AN, Thompson RH, editors. Nutritional growth restriction and the nervous system. The molecular basis of neuropathology. 1981. 2nd ed. London: Edward Arnold.30. Coulthard MG, Hey EN. Effect of varying water intake on renal function in healthy preterm babies. Arch Dis Child. 1985. 60:614–620.31. Cowett RM, Oh W, Schwartz R. Persistent glucose production during glucose infusion in the neonate. J Clin Invest. 1983. 71:467–475.32. Ditzenberger GR, Collins SD, Binder N. Continuous insulin intravenous infusion therapy for VLBW infants. J Perinat Neonatal Nurs. 1999. 13:70–82.33. Louaib D, Lachassinne E, Nathanson M, Muller MH, Sauvion S, Gaudelus J. Hyperglycemia revealing neonatal infection. Arch Pediatr. 1994. 1:819–821.34. Koivisto M, Peltoniemi OM, Saarela T, Tammela O, Jouppila P, Hallman M. Blood glucose level in preterm infants after antenatal exposure to glucocorticoid. Acta Paediatr. 2007. 96:664–668.35. Simmons RA, Templeton LJ, Gertz SJ. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes. 2001. 50:2279–2286.36. Blanco CL, Baillargeon JG, Morrison RL, Gong AK. Hyperglycemia in extremely low birth weight infants in a predominantly Hispanic population and related morbidities. J Perinatol. 2006. 26:737–741.37. Collins JW Jr, Hoppe M, Brown K, Edidin DV, Padbury J, Ogata ES. A controlled trial of insulin infusion and parenteral nutrition in extremely low birth weight infants with glucose intolerance. J Pediatr. 1991. 118:921–927.38. Poindexter BB, Karn CA, Denne SC. Exogenous insulin reduces proteolysis and protein synthesis in extremely low birth weight infants. J Pediatr. 1998. 132:948–953.39. Gibson AT, Carney S, Cavazzoni E, Wales JK. Neonatal and post-natal growth. Horm Res. 2000. 53:42–49.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Optimal Ventilatory Strategies in Preterm Infants: Permissive Hypercapnia
- Meconium Obstruction Syndrome in Two Extremely Low Birth Weight Infants
- Clinical Study of Prematurity and Low Birth Weight Infants
- Glucose Homeostasis Disorders in Premature Infants
- Neurodevelopmental Outcomes of Extremely Preterm Infants