J Korean Med Sci.
2004 Jun;19(3):437-446. 10.3346/jkms.2004.19.3.437.
Effect of Thermal Preconditioning Before Excimer Laser Photoablation
- Affiliations
-
- 1Department of Ophthalmology, College of Medicine, Seoul National University, Seoul, Korea.
- 2Department of Ophthalmology, Chung-Ang University Hospital, Seoul, Korea. cauheye@hananet.net
- 3Department of Ophthalmology, Dong-A University Hospital, Busan, Korea.
- 4Ilchun Molecular Medicine Institute and Department of Biochemistry, College of Medicine, Seoul National University, Seoul, Korea.
- 5Department of Ophthalmology, Kangbuk Samsung Hospital, Sungkyunkwan University, Seoul, Korea.
- KMID: 1786825
- DOI: http://doi.org/10.3346/jkms.2004.19.3.437
Abstract
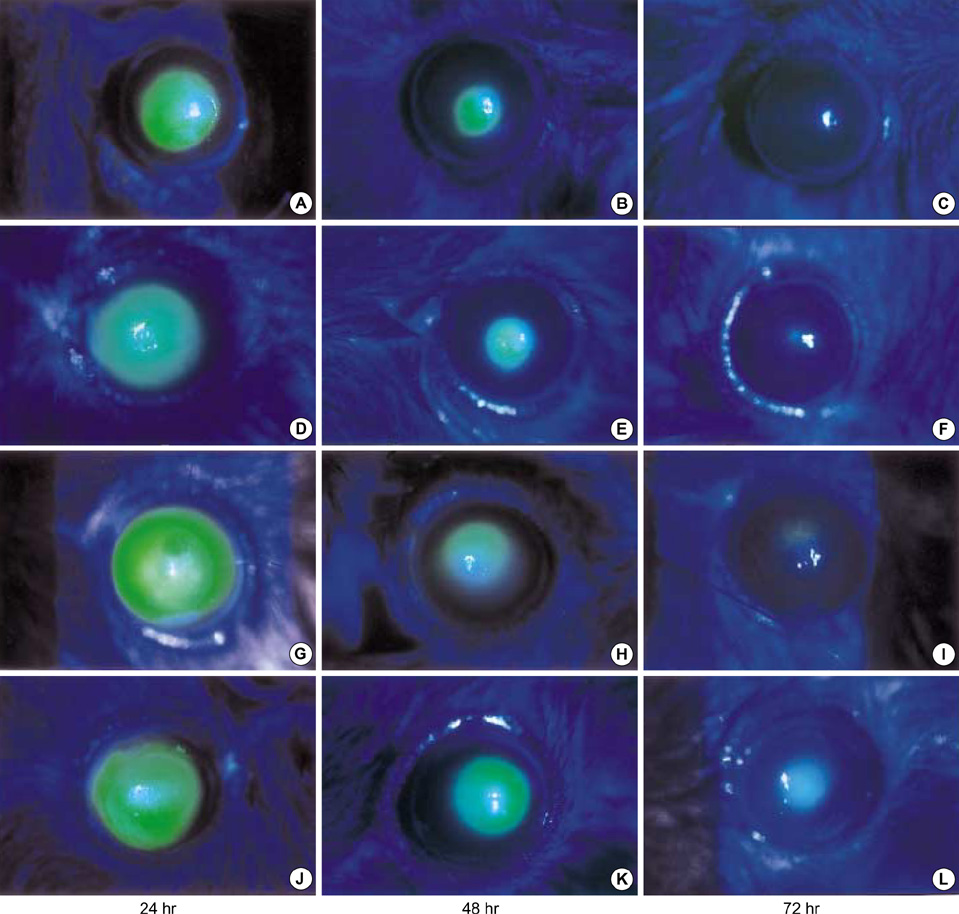
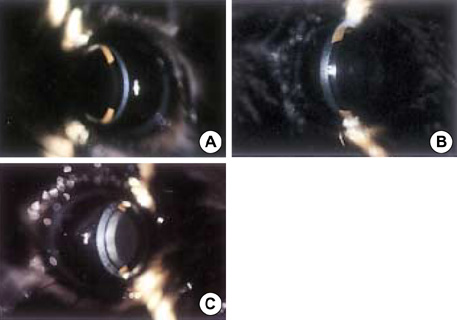
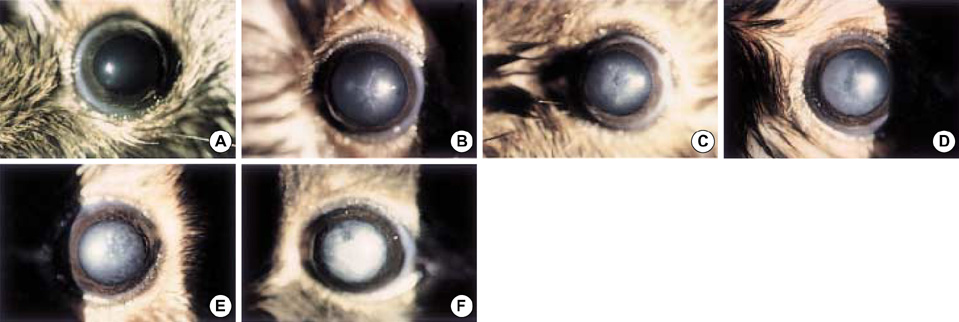
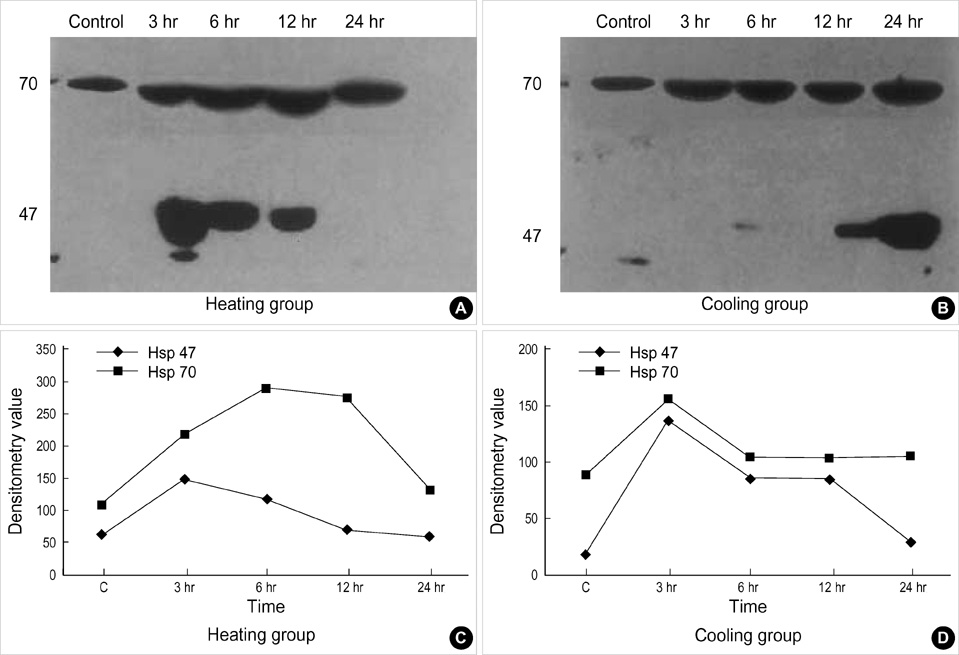
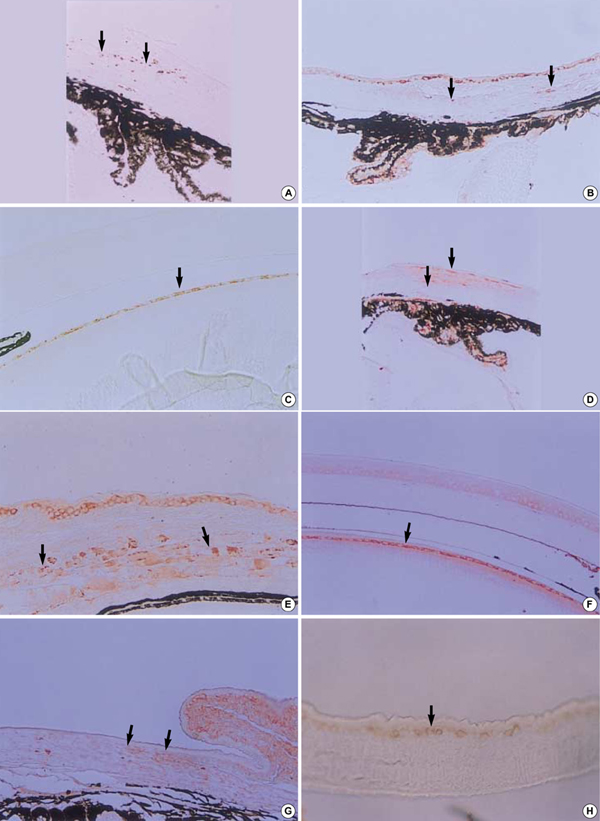
- The purposes of this study were to assess the expression patterns of heat shock proteins (Hsps), after eyeball heating or cooling, and to elucidate their relationships with corneal wound healing and intraocular complications after excimer laser treatment. Experimental mice were grouped into three according to local pretreatment type: heating, cooling, and control groups. The preconditioning was to apply saline eyedrops onto the cornea prior to photoablation. Following photoablation, we evaluated corneal wound healing, corneal opacity and lens opacity. Hsp expression patterns were elucidated with Western blot and immunohistochemical staining. The heating and cooling groups recovered more rapidly, and showed less corneal and lens opacity than the control group. In the heating and cooling groups, there were more expressions of Hsps in the cornea and lens than in the control group. These results were confirmed in the Hsp 70.1 knockout mouse model. Our study showed that Hsps were induced by the heating or cooling preconditioning, and appeared to be a major factor in protecting the cornea against serious thermal damage. Induced Hsps also seemed to play an important role in rapid wound healing, and decreased corneal and lens opacity after excimer laser ablation.
Keyword
MeSH Terms
-
Animals
Blotting, Western
Cornea/pathology
Heat
Heat-Shock Proteins/biosynthesis
Heat-Shock Proteins 70/genetics
Immunohistochemistry
Keratectomy, Photorefractive, Excimer Laser/*methods
*Lasers
Lens, Crystalline/pathology
Mice
Mice, Inbred C57BL
Mice, Knockout
Support, Non-U.S. Gov't
Temperature
Time Factors
Wound Healing
Figure
Reference
-
1. Marshall J, Trokel SL, Rothery S, Krueger RR. Long-term healing of the central cornea after photorefractive keratectomy using an excimer laser. Ophthalmology. 1988. 95:1411–1421.
Article2. Langenbucher A, Seitz B, Kus MM, Nauman GO. Thermal effects in excimer laser trephination of the cornea. Graefes Arch for Clin Exp Ophthalmol. 1996. 234:Suppl 1. S142–S148.
Article3. Bende T, Seiler T, Wollensak J. Corneal thermal gradients. Grafes Arch for Clin Exp Ophthalmol. 1988. 226:277–280.4. Tsubota K, Toda I, Itoh S. Reduction of subepithelial haze after photorefractive keratectomy by cooling the cornea. Am J Ophthalmol. 1993. 115:820–821.
Article5. Park WC, Tseng SCG. Temperature cooling reduces keratocyte death in excimer laser ablated corneal and skin wounds. Invest Ophthmol Vis Sci. 1988. 39:Suppl 1. 2062.6. Tanaka Y, Kobayashi K, Kita M, Masuda H, Kinoshita S, Nakata K, Imanishi J. Expression of 47 kDa heat shock protein (HSP47) during development of mouse cornea. Exp Eye Res. 1996. 63:383–393.
Article7. Barbe MF, Tytell M, Gower DJ, Welch WJ. Hyperthermia protects against light damage in the rat retina. Science. 1988. 241:1817–1820.
Article8. Laios E, Rebeyka IM, Prody CA. Characterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol Cell Biochem. 1997. 173:153–159.9. Tamm ER, Russell P, Johnson DH, Piatigorsky J. Human and monkey trabecular meshwork accumulate α-crystallin in response to heat shock and oxidative stress. Invest Ophthalmol Vis Sci. 1996. 37:2402–2413.10. Ellis J. Proteins as molecular chaperones. Nature. 1987. 328:378–379.
Article11. Hendrick JP, Hartl FU. Molecular chaperone functions of heat-shock proteins. Annu Rev Biochem. 1993. 62:349–384.
Article12. Lindquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988. 22:631–677.13. Morimoto RI, Tissieres A, Georgopoulos C. Morimoto RI, Tissieres A, Georgopou;os C, editors. Progress and perspectives on the biology of heat shock proteins and molecular chaperones. Biology of heat shock proteins and molecular chaperones. 1994. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press;1–30.14. Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992. 355:33–45.
Article15. Polla BS. A role for heat shock proteins in inflammation? Immunol Today. 1988. 9:134–137.
Article16. Jakob U, Buchner J. Assisting spontaneity: the role of hsp90 and small hsp as molecular chaperones. Trends Biochem Sci. 1994. 19:205–211.17. Hirayoshi K, Kudo H, Tacheshi H, Nakai A, Iwamatsu A, Yamada KM, Nagata K. HSP 47: a tissue-specific transformation-sensitive, collagen-binding heat shock protein of chicken embryo fibroblasts. Mol Cell Biol. 1991. 11:4036–4044.18. Brown CR, Martin RL, Hansen WJ, Beckmann RP, Welch WJ. The constitutive and stress inducible forms of hsp 70 exhibit functional similarities and interact with one another in an ATP-dependent fashion. J Cell Biol. 1993. 120:1101–1112.
Article19. Muchowski PJ, Valdez MM, Clark JI. AlphaB-crystallin selectively targets intermediate filament proteins during thermal stress. Invest Ophthalmol Vis Sci. 1999. 40:951–958.20. Kim YM, de Vera ME, Watkins SC, Billar TR. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor alpha induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997. 272:1402–1411.21. Bellmann K, Wenz A, Radons J, Burkart V, Kleemann R, Kolb H. Heat shock induces resistance in rat pancreatic islet cells against nitric oxide, oxygen radicals and streptozotocin toxicity in vitro. J Clin Invest. 1995. 95:2840–2845.
Article22. Laios E, Rebeyka M, Prody CA. Characterization of cold-induced heat shock protein expression in neonatal rat cardiomyocytes. Mol Cell Biochem. 1997. 173:153–159.23. Koroshetz WJ, Bonventre JV. Heat shock response in the central nervous system. Experientia. 1994. 50:1085–1091.
Article24. Polla BS, Bonventre JV, Krane SM. 1,25-dihydroxyvitamin D3 increases the toxicity of hydrogen peroxide in the human monocytic line U937: Role of calcium and heat shock. J Cell Biol. 1988. 107:373–380.25. Spitz DR, Dewey WC, Li GC. Hydrogen peroxide or heat shock induces resistance to hydrogen peroxide in Chinese hamster fibroblast. J Cell Physiol. 1987. 131:364–373.26. Li GC, Li L, Liu YK, Mak JY, Chen LL, Lee WM. Thermal response of rat fibroblasts transfected with the human 70-kDa heat shock protein-encoding gene. Proc Natl Acad Sci USA. 1991. 88:1681–1685.27. Currie RW, Tanguay RM. Analysis of RNA for transcripts for catalase and SP71 in rat hearts after in vivo hyperthermia. Biochem Cell Biol. 1991. 69:375–382.
Article28. Suzuki K, Sawa Y, Kanada Y, Ichikawa H, Shirakura R, Masuda H. In vivo gene transfection with heat shock protein 70 enhances myocardial tolerance to ischemia-reperfusion injury in rat. J Clin Invest. 1997. 99:1645–1650.
Article29. Yellon DM, Latchman DS. Stress proteins and myocardial protection. J Mol Cell Cardiol. 1992. 24:113–124.30. Yamaguchi K, Barbe MF, Brown JR, Tytell M. Induction of stress (heat shock) protein 70 and its mRNA in rat corneal epithelium by hyperthermia. Curr Eye Res. 1990. 9:913–918.
Article31. Pirie A. A light-catalysed reaction in the aqueous humor of the eye. Nature. 1965. 205:500–501.32. Stadtman ER. Oxidations of proteins by mixed function oxidation systems.: Implications in protein turnover, aging and neutrophil function. Trends Biochem Sci. 1986. 11:11–12.33. Nagata K, Saga S, Yamada KM. A major collagen-binding protein of chick embryo fibroblast is a novel heat shock protein. J Cell Biol. 1986. 103:223–229.34. Nagata K, Yamada KM. Phosphorylation and transformation sensitivity of a major collagen binding protein of fibroblasts. J Biol Chem. 1986. 261:7531–7536.35. Saga S, Nakata K, Chen WT, Yamada KM. pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J Cell Biol. 1987. 105:517–527.
Article36. Nakai A, Hirayoshi K, Nagata K. Transformation of BALB/3T3 cells by Simian virus 40 causes a decreased synthesis of a collagen-binding heatshock protein (HSP 47). J Biol Chem. 1990. 265:992–999.37. Takechi H, Hirayoshi K, Nakai A, Kudo H, Saga S, Nakata K. Molecular cloning of a mouse 47-kDa heat shock protein (HSP47), a collagen binding stress protein, and its expression during the differentiation of F9 teratocarcinoma cells. Eur J Biochem. 1992. 206:323–329.38. Natsume T, Koide T, Yokota S, Hirayoshi K, Nagata K. Interactions between collagen-binding stress protein HSP 47 and collagen. Analysis of kinetic parameters by surface plasmon resonance biosensor. J Biol Chem. 1994. 269:31224–31228.39. Masuda H, Fukumoto M, Hirayoshi K, Nagata K. Coexpression of the collagen-binding stress protein HSP47 gene and the α1(I) and α1(III) collagen genes in carbon tetrachloride-induced rat liver fibrosis. J Clin Invest. 1994. 94:2481–2488.40. Sarthy V. Collagen IV mRNA expression during development of the mouse retina: an in situ hybridization study. Invest Ophthalmol Vis Sci. 1993. 34:145–152.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Excimer Laser Phototherapeutic Keratectomy for Experimental Fungal Keratitis
- A Histological Comparative Analysis of Thermal Side Effects of Excimer Laser Versus Holmium : YAG Laser in the Human Articular Cartilage
- Corneal Endothelial Permeability after Deep Excimer Laser Ablation
- Corneal Endothelial Damage after Deep Excimer Laser Ablation
- Excimer laser Radial Keratotomy for Correction of Myopia and Astigmatism: Preliminary Report