Yonsei Med J.
2007 Dec;48(6):1020-1027. 10.3349/ymj.2007.48.6.1020.
Sulindac Prevents Esophageal Adenocarcinomas Induced by Gastroduodenal Reflux in Rats
- Affiliations
-
- 1Department of Gastroenterology, Dongguk University College of Medicine, Kyongju, Korea.
- 2Department of Pathology, Dongguk University College of Medicine, Kyongju, Korea. taejung@mail.dongguk.ac.kr
- 3Department of General Surgery, Dongguk University College of Medicine, Kyongju, Korea.
- KMID: 1786219
- DOI: http://doi.org/10.3349/ymj.2007.48.6.1020
Abstract
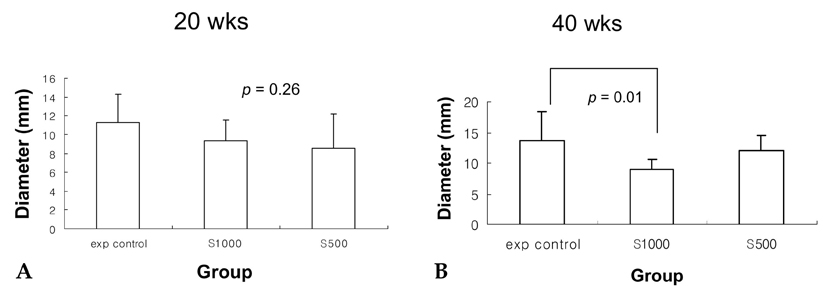
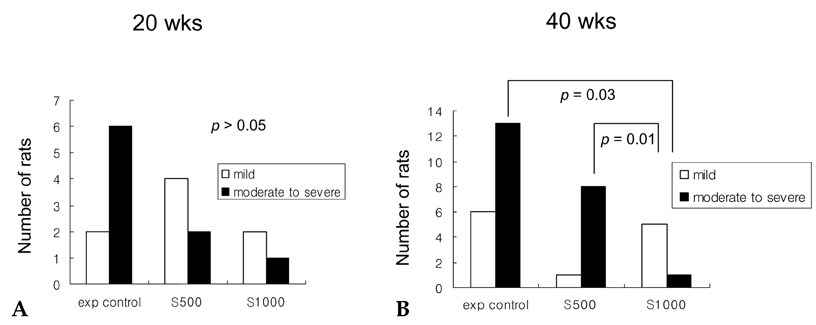
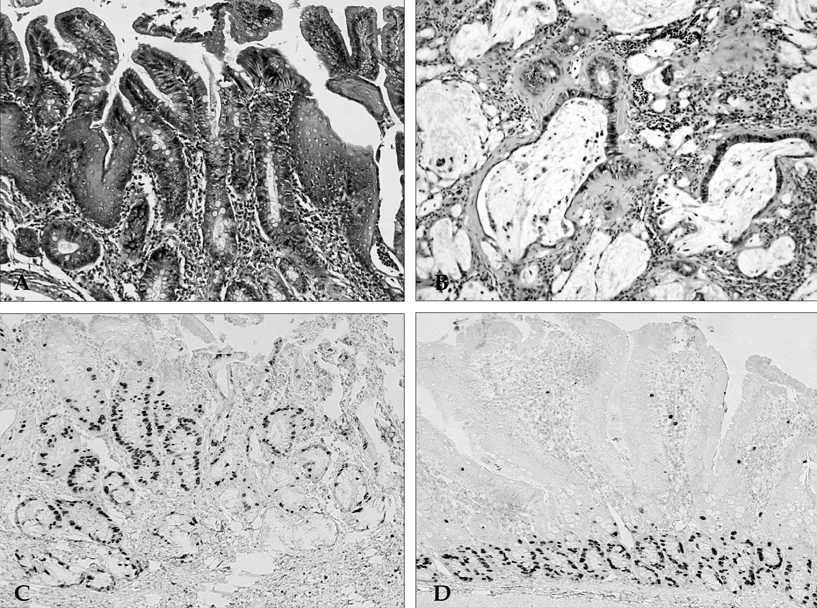
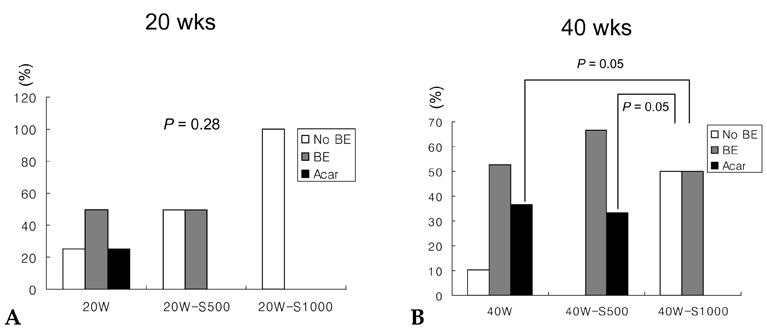
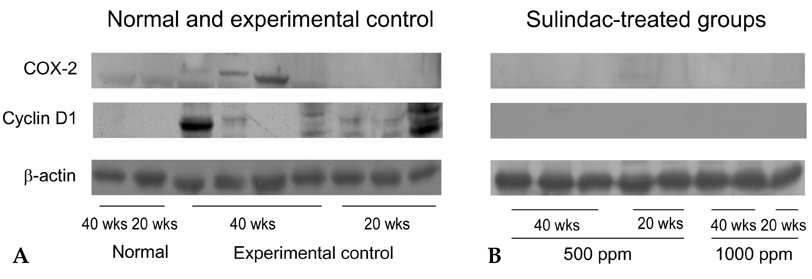
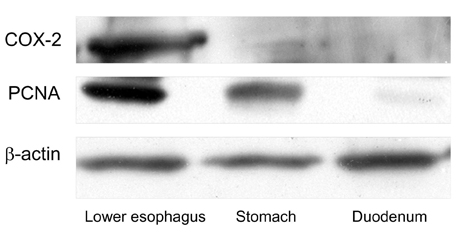
- PURPOSE: It is known that cyclooxygenase (COX)-2 expression is increased in Barrett's esophagus and esophageal adenocarcinomas. We studied COX-2 expression and the effect sulindac has on the genesis of Barrett's esophagus and adenocarcinoma in rats undergoing esophagogastroduodenal anastomosis (EGDA). MATERIALS AND METHODS: Fifty-one rats were divided into a control group (n=27), a 500ppm sulindac-treated group (n=15) and 1000 ppm sulindac-treated group (n=9). Randomly selected rats were killed by diethyl ether inhalation at 20 and 40 weeks after surgery. RESULTS: At 40 weeks, rats treated with 1000 ppm sulindac showed narrower esophageal diameter and milder inflammation than the control rats. At 40 weeks, the incidence of Barrett's esophagus was similar between control and sulindac-treated groups, but the incidence of adenocarcinoma was significantly lower in the 1000ppm sulindac-treated group than either the control or 500 ppm sulindac-treated groups. COX-2 was significantly increased in the lower esophagus of control rats killed at 40 weeks. Cyclin D1 expression was negligible in the sulindac- treated group compared with the control group. CONCLUSION: We suggest that the chemopreventive effect of sulindac is related to decreased COX-2 and cyclin D1 expression, which may be influenced by reduced inflammation.
MeSH Terms
-
Adenocarcinoma/etiology/metabolism/*prevention & control
Animals
Antineoplastic Agents/therapeutic use
Barrett Esophagus/etiology/metabolism/prevention & control
Blotting, Western
Cyclin D1/metabolism
Cyclooxygenase 2/metabolism
Duodenogastric Reflux/*complications
Esophageal Neoplasms/etiology/metabolism/*prevention & control
Immunohistochemistry
Male
Proliferating Cell Nuclear Antigen/metabolism
Rats
Rats, Sprague-Dawley
Sulindac/*therapeutic use
Figure
Reference
-
1. Blot WJ, Devesa SS, Kneller RW, Fraumeni JF Jr. Rising incidence of adenocarcinoma of the esophagus and gastric cardia. JAMA. 1991. 265:1287–1289.2. Pera M, Cameron AJ, Trastek VF, Carpenter HA, Zinsmeister AR. Increasing incidence of adenocarcinoma of the esophagus and esophagogastric junction. Gastroenterology. 1993. 104:510–513.3. Barrett NR. The lower esophagus lined by columnar epithelium. Surgery. 1957. 41:881–894.4. Paull A, Trier JS, Dalton MD, Camp RC, Loeb P, Goyal RK. The histologic spectrum of Barrett's esophagus. N Engl J Med. 1976. 295:476–480.5. Winters C Jr, Spurling TJ, Chobanian SJ, Curtis DJ, Esposito RL, Hacker JF 3rd, et al. Barrett's esophagus. A prevalent, occult complication of gastroesophageal reflux disease. Gastroenterology. 1987. 92:118–124.6. Reid BJ. Barrett's esophagus and esophageal adenocarcinoma. Gastroenterol Clin North Am. 1991. 20:817–834.7. Kauer WK, Peters JH, DeMeester TR, Ireland AP, Bremner CG, Hagen JA. Mixed reflux of gastric and duodenal juices is more harmful to the esophagus than gastric juice alone. The need for surgical therapy re-emphasized. Ann Surg. 1995. 222:525–533.8. Zhang F, Altorki NK, Wu YC, Soslow RA, Subbaramaiah K, Dannenberg AJ. Duodenal reflux induces cyclooxygenase-2 in the esophageal mucosa of rats: evidence for involvement of bile acids. Gastroenterology. 2001. 121:1391–1399.9. Shirvani VN, Ouatu-Lascar R, Kaur BS, Omary MB, Triadafilopoulos G. Cyclooxygenase 2 expression in Barrett's esophagus and adenocarcinoma: Ex vivo induction by bile salts and acid exposure. Gastroenterology. 2000. 118:487–496.10. Jang TJ, Min SK, Bae JD, Jung KH, Lee JI, Kim JR, et al. Expression of cyclooxygenase 2, microsomal prostaglandin E synthase 1, and EP receptors is increased in rat oesophageal squamous cell dysplasia and Barrett's metaplasia induced by duodenal contents reflux. Gut. 2004. 53:27–33.11. Herschman HR. Prostaglandin synthase 2. Biochim Biophys Acta. 1996. 1299:125–140.12. DeWitt DL. Prostaglandin endoperoxide synthase: regulation of enzyme expression. Biochim Biophys Acta. 1991. 1083:121–134.13. Jones MK, Wang H, Peskar BM, Levin E, Itani RM, Sarfeh IJ, et al. Inhibition of angiogenesis by nonsteroidal anti-inflammatory drugs: insight into mechanisms and implications for cancer growth and ulcer healing. Nat Med. 1999. 5:1418–1423.14. Tsujii M, Kawano S, Tsuji S, Sawaoka H, Hori M, DuBois RN. Cyclooxygenase regulates angiogenesis induced by colon cancer cells. Cell. 1998. 93:705–716.15. Tsujii M, Kawano S, DuBois RN. Cyclooxygenase-2 expression in human colon cancer cells increases metastatic potential. Proc Natl Acad Sci U S A. 1997. 94:3336–3340.16. Tsujii M, DuBois RN. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell. 1995. 83:493–501.17. Kune GA, Kune S, Watson LF. Colorectal cancer risk, chronic illnesses, operations, and medications: case control results from the Melbourne Colorectal Cancer Study. Cancer Res. 1988. 48:4399–4404.18. Coogan PF, Rosenberg L, Palmer JR, Strom BL, Zauber AG, Stolley PD, et al. Nonsteroidal anti-inflammatory drugs and risk of digestive cancers at sites other than the large bowel. Cancer Epidemiol Biomarkers Prev. 2000. 9:119–123.19. Langman MJ, Cheng KK, Gilman EA, Lancashire RJ. Effect of anti-inflammatory drugs on overall risk of common cancer: case-control study in general practice research database. BMJ. 2000. 320:1642–1646.20. Lindblad M, Lagergren J, García Rodríguez LA. Nonsteroidal anti-inflammatory drugs and risk of esophageal and gastric cancer. Cancer Epidemiol Biomarkers Prev. 2005. 14:444–450.21. Corley DA, Kerlikowske K, Verma R, Buffler P. Protective association of aspirin/NSAIDs and esophageal cancer: a systematic review and meta-analysis. Gastroenterology. 2003. 124:47–56.22. Anderson LA, Johnston BT, Watson RG, Murphy SJ, Ferguson HR, Comber H, et al. Nonsteroidal anti-inflammatory drugs and the esophageal inflammation-metaplasia-adenocarcinoma sequence. Cancer Res. 2006. 66:4975–4982.23. Oyama K, Fujimura T, Ninomiya I, Miyashita T, Kinami S, Fushida S, et al. A COX-2 inhibitor prevents the esophageal inflammation-metaplasia-adenocarcinoma sequence in rats. Carcinogenesis. 2005. 26:565–570.24. Buttar NS, Wang KK, Leontovich O, Westcott JY, Pacifico RJ, Anderson MA, et al. Chemoprevention of esophageal adenocarcinoma by COX-2 inhibitors in an animal model of Barrett's esophagus. Gastroenterology. 2002. 122:1101–1112.25. Chen X, Yang G, Ding WY, Bondoc F, Curtis SK, Yang CS. An esophagogastroduodenal anastomosis model for esophageal adenocarcinogenesis in rats and enhancement by iron overload. Carcinogenesis. 1999. 20:1801–1808.26. Oshima M, Dinchuk JE, Kargman SL, Oshima H, Hancock B, Kwong E, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase-2 (COX-2). Cell. 1996. 87:803–809.27. Kawamori T, Rao CV, Seibert K, Reddy BS. Chemopreventive activity of celecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res. 1998. 58:409–412.28. Zimmermann KC, Sarbia M, Weber AA, Borchard F, Gabbert HE, Schror K. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res. 1999. 59:198–204.29. Wilson KT, Fu S, Ramanujam KS, Meltzer SJ. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett's esophagus and associated adenocarcinoma. Cancer Res. 1998. 58:2929–2934.30. Li Z, Shimada Y, Kawabe A, Sato F, Maeda M, Komoto I, et al. Suppression of N- nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by JTE-522, a selective COX-2 inhibitor. Carcinogenesis. 2001. 22:547–551.31. Soslow RA, Remotti H, Baergen RN, Altorki NK. Suppression of apoptosis does not foster neoplastic growth in Barrett's esophagus. Mod Pathol. 1999. 12:239–250.32. Jang TJ, Cho MY. Cyclooxygenase-2 expression and cell proliferation are increased in MUC2-positive area of columnar-lined esophagus. Pathol Int. 2005. 55:546–549.33. Koppert LB, Wijnhoven BP, van Dekken H, Tilanus HW, Dinjens WN. The molecular biology of esophageal adenocarcinoma. J Surg Oncol. 2005. 92:169–190.34. Kim JS, Baek SJ, Sali T, Eling TE. The conventional nonsteroidal anti-inflammatory drug sulindac sulfide arrests ovarian cancer cell growth via the expression of NAG-1/MIC-1/GDF-15. Mol Cancer Ther. 2005. 4:487–493.35. Takada Y, Bhardwaj A, Potdar P, Aggarwal BB. Nonsteroidal anti-inflammatory agents differ in their ability to suppress NF-kappa B activation, inhibition of expression of cyclooxygenase-2 and cyclin D1, and abrogation of tumor cell proliferation. Oncogene. 2004. 23:9247–9258.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Understanding the Rome IV: Esophageal Disorders
- Is Gastroesophageal Reflux Disease and Achalasia Coincident or Not?
- Effect of Lactacystin on the Sulindac-Induced Apoptosis Mechanisms in HT-29 Cells
- Effects of a mixture of Citri Pericarpium and Scutellariae Radix on acute reflux esophagitis in rats
- Esophageal Manometry and 24 Hours Ambulatory Esophageal pH Monitoring in Patients with Globus Pharyngeus