Yonsei Med J.
2007 Dec;48(6):1001-1008. 10.3349/ymj.2007.48.6.1001.
A Prospective, Multicenter, Open-label Trial of Zoledronic Acid in Patients with Hormone Refractory Prostate Cancer
- Affiliations
-
- 1Department of Urology, Yonsei University College of Medicine, Seoul, Korea. sjhong346@yuhs.ac
- 2Department of Urology, Sungkyunkwan University, Seoul, Korea.
- 3Department of Urology, Ulsan University, Seoul, Korea.
- KMID: 1786216
- DOI: http://doi.org/10.3349/ymj.2007.48.6.1001
Abstract
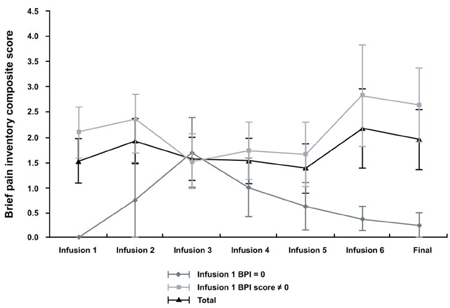
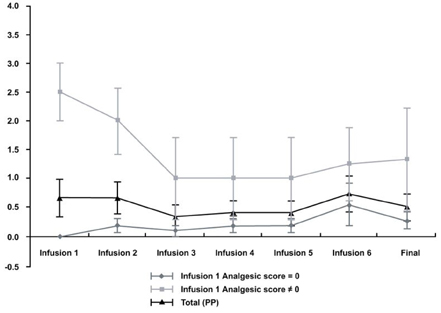
- PURPOSE: The short-term safety and efficacy of zoledronic acid for the treatment of skeletal metastasis was evaluated in patients with hormone-refractory prostate cancer. PATIENTS AND METHODS: A total of 19 hormone-refractory prostate cancer patients with bone metastases were enrolled. All patients received up to six infusions of zoledronic acid (4mg, given intravenously over 15 minutes, every 3-4 weeks). Safety was assessed by monitoring a`dverse events and serum creatinine levels. Efficacy was assessed by monitoring skeletal-related events, brief pain inventory score, quality of life score, type of pain medication, and analgesic score. Mean age of patients was 67.3 years (46-86 years), mean time from diagnosis of bone metastases was 27.6 months (0-117 months), and mean time from diagnosis of hormone-refractory disease was 7.5 months (0-26 months). RESULTS: There was no clinically significant change in serum creatinine levels. Eleven adverse events (musculoskeletal disorders and systemic disorders) in 8 patients were classed as having a possible relationship to study drug. Fifteen patients completed six courses of zoledronic acid infusion. There were no significant changes in the brief pain inventory composite scores, quality of life questionnaire scores or analgesic score. No new skeletal-related events developed during the treatment period. CONCLUSION: Zoledronic acid administered in this study as a 15-minute infusion demonstrated an acceptable and well-known safety profile in patients with refractory prostate cancer with bone metastases. However, prospective placebo- controlled clinical trials are required to elucidate the efficacy of zoledronic acid.
MeSH Terms
-
Aged
Aged, 80 and over
Antineoplastic Agents, Hormonal/therapeutic use
Bone Density Conservation Agents/adverse effects/therapeutic use
Bone Neoplasms/drug therapy/secondary
Creatinine/blood
Diphosphonates/adverse effects/*therapeutic use
Drug Resistance, Neoplasm
Humans
Imidazoles/adverse effects/*therapeutic use
Male
Middle Aged
Prospective Studies
Prostatic Neoplasms/*drug therapy/pathology
Quality of Life
Treatment Outcome
Figure
Cited by 1 articles
-
Exponential Rise in Prostate-Specific Antigen (PSA) during Anti-Androgen Withdrawal Predicts PSA Flare after Docetaxel Chemotherapy in Patients with Castration-Resistant Prostate Cancer
Kyung Seok Han, Sung Joon Hong
Yonsei Med J. 2015;56(2):368-374. doi: 10.3349/ymj.2015.56.2.368.
Reference
-
1. Ferlay J, Bray F, Pisani P, Parkin DM. GLOBOCAN 2000: cancer incidence, mortality and prevalence worldwide. 2001. version 1.0. Lyon: IARC Press;IARC Cancer Base no. 5.2. Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001. 27:165–176.3. Siddiqui NA, Shetty KR, Duthie EH Jr. Osteoporosis in older men: discovering when and how to treat it. Geriatrics. 1999. 54:20–22. 27–28. 30passim.4. Smith MR. Diagnosis and management of treatment-related osteoporosis in men with prostate carcinoma. Cancer. 2003. 97:3 Suppl. 789–795.5. Smith MR, McGovern FJ, Fallon MA, Schoenfeld D, Kantoff PW, Finkelstein JS. Low bone mineral density in hormone-naïve men with prostate carcinoma. Cancer. 2001. 91:2238–2245.6. Wei JT, Gross M, Jaffe CA, Gravlin K, Lahaie M, Faerber GJ, et al. Androgen deprivation therapy for prostate cancer results in significant loss of bone density. Urology. 1999. 54:607–611.7. Khosla S, Melton LJ 3rd, Atkinson EJ, O'Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001. 86:3555–3561.8. Smith MR. Treatment-related osteoporosis in men with prostate cancer. Clin Cancer Res. 2006. 12:6315S–6319S.9. Morote J, Martinez E, Trilla E, Esquena S, Abascal JM, Encabo G, et al. Osteoporosis during continuous androgen deprivation: influence of the modality and length of treatment. Eur Urol. 2003. 44:661–665.10. Weinfurt KP, Li Y, Castel LD, Saad F, Timbie JW, Glendenning GA, et al. The significance of skeletal-related events for the health-related quality of life of patients with metastatic prostate cancer. Ann Oncol. 2005. 16:579–584.11. Cheer SM, Noble S. Zoledronic acid. Drugs. 2001. 61:799–806.12. Gucalp R, Ritch P, Wiernik PH, Sarma PR, Keller A, Richman SP, et al. Comparative study of pamidronate disodium and etidronate disodium in the treatment of cancer-related hypercalcemia. J Clin Oncol. 1992. 10:134–142.13. Ralston SH, Gallacher SJ, Patel U, Dryburgh FJ, Fraser WD, Cowan RA, et al. Comparison of three intravenous bisphosphonates in cancer-associated hypercalcaemia. Lancet. 1989. 2:1180–1182.14. Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0. 2003. March. 31. DCTC, NCI, NIH, DHHS;(http://ctep.cancer.gov). Publish Date: August 9, 2006.15. Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994. 23:129–138.16. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993. 11:570–579.17. Tong D, Gillick L, Hendrickson FR. The palliation of symptomatic osseous metastases: final results of the Study by the Radiation Therapy Oncology Group. Cancer. 1982. 50:893–899.18. Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002. 2:584–593.19. Saad F. Preventing bone complications in patients with prostate cancer: the emerging role of zoledronic acid. Eur Urol Suppl. 2004. 3:25–33.20. Saad F, Karakiewicz P, Perrotte P. The role of bisphosphonates in hormone-refractory prostate cancer. World J Urol. 2005. 23:14–18.21. Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004. 96:879–882.22. Body JJ. Clinical research update: zoledronate. Cancer. 1997. 80:8 Suppl. 1699–1701.23. Lipton A, Berenson JR. Bisphosphonate treatment of lytic bone metastases. Drugs Aging. 1999. 14:241–246.24. Chen T, Berenson J, Vescio R, Swift R, Gilchick A, Goodin S, et al. Pharmacokinetics and pharmacodynamics of zoledronic acid in cancer patients with bone metastases. J Clin Pharmacol. 2002. 42:1228–1236.25. Guarneri V, Donati S, Nicolini M, Giovannelli S, D'Amico R, Conte PF. Renal safety and efficacy of i.v. bisphosphonates in patients with skeletal metastases treated for up to 10 Years. Oncologist. 2005. 10:842–848.26. Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002. 94:1458–1468.27. Saad F, Olsson C, Schulman CC. Skeletal morbidity in men with prostate cancer: quality-of-life considerations throughout the continuum of care. Eur Urol. 2004. 46:731–740.28. Migliorati CA, Siegel MA, Elting LS. Bisphosphonate-associated osteonecrosis: a long-term complication of bisphosphonate treatment. Lancet Oncol. 2006. 7:508–514.29. Carroll PR, Altwein J, Brawley O, Cockett A, Cooperberg M, Hirao Y, et al. Denis L, Bartsch G, Khoury S, Murai M, Partin A, editors. Management of disseminated prostate cancer. Prostate Cancer: 3rd International Consultation on Prostate Cancer-Paris. 2003. Paris: Health publications;251–284.30. Ryan CW, Huo D, Demers LM, Beer TM, Lacerna LV. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006. 176:972–978.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adjuvant Effect of IV Clodronate on the Delay of Bone Metastasis in High-Risk Prostate Cancer Patients: A Prospective Study
- Dramatic Decline of PSA and Symptom Improvement after Estramustine Withdrawal in a Hormone-refractory Prostate Cancer Patient
- The Change of Serum Insulin-Like Growth Factor Binding Protein 3 (IGFBP-3) in the Diagnosis and Management of Prostate Cancer
- Efficacy of zoledronic acid in older prostate cancer patients undergoing androgen deprivation therapy
- Is High-Dose Leuprorelin Acetate Effective and Safe in Asian Men with Prostate Cancer? An Open-Label, Non-Comparative, Multi-Center Clinical Trial